Apart from optical properties such as color and hiding, certain pigments are used in protective coatings to increase their corrosion resistance properties. The anticorrosive pigments used in conjunction with suitable binder in protective coatings systems play a major role in protection of metals from corrosion.
Based on their mode of action to prevent corrosion, anticorrosive pigments may be classified in the following types:
Active pigments (inhibitive pigments): These are anticorrosive pigments with chemical and/or electrochemical action. When water penetrates the coating film, these pigments release soluble passivating ions that help in corrosion protection by promoting the formation of a protective layer at the metal surface, or by polarizing or inhibiting either or both of the two electrochemical reactions of the corrosion process, known as the cathodic and anodic reactions. Another mechanism of action of active pigments is neutralization of corrosive substances such as sulfates, acids and chlorides. Solubility and reactivity are the critical parameters of active pigments.
Barrier pigments: These act by reinforcing the paint film and reducing its permeability to corrosive agents. Generally, they are chemically inert pigments with a platelet-like or lamellar particle shape. This allows them to form a wall of flat particles stacked within a paint film, and therefore, water and electrolytes must take an extended indirect route through the paint film to the substrate, as described in Figure 3.7, thus increasing the barrier properties of the coating film.
|
Figure 3.7: Demonstration of the barrier effect when using lamellar pigments |
Sacrificial pigments: These are a special type of active pigments that act by cathodic protection when applied to ferrous substrates. They are metallic pigments that are higher in the electromotive series of metals than the metal substrate to be protected. Under corrosive conditions, sacrificial pigments, being more reactive than the substrate, act as the anode of an electrochemical corrosion cell, drawing attack on themselves and protecting the substrate metal. Therefore, this mechanism is also term cathodic protection. The coating must be formulated to yield good electrical contact between substrate metal and sacrificial pigment particles.
Some commercially important barrier pigments are micaceous iron oxide (Section 3.3.3.1) and aluminum flakes (Section 3.3.5.1). The only sacrificial pigment of commercial importance is zinc dust (Section 3.3.5.2).
 16 ноября, 2015
16 ноября, 2015  Pokraskin
Pokraskin 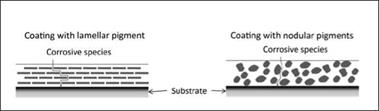
 Опубликовано в рубрике
Опубликовано в рубрике 