The term amino resins is used to describe a very important and versatile group of thermoset polymers derived from nitrogen-containing compounds, most typically, melamine and urea. Amino resins (also known as aminoplasts) are widely used in a number of non-coating thermoset applications. In coatings, they are used mainly as cross-linkers, and not as primary film forming resins. The introduction of amino cross-linking agents facilitated coating formulators in designing products with improved thermo-mechanical and resistance properties, because before their introduction, most coatings were physically drying or oxidative curing systems, with a few exceptions of thermosetting coatings based on pheno — plasts. Amino resins helped overcome some inherent limitations of
phenolic resins, such as high baking temperatures and discoloration, and therefore they emerged as very versatile cross-linkers for thermoset coatings.
Amino resins are thermosetting polymers that are produced from nitrogen-containing compounds with reactive amino or amido groups that can readily be converted to corresponding methylol compounds upon reaction with formaldehyde. Urea, melamine, benzoguanamine and glycoluril are among the important amino compounds used to derive aminoplasts suitable for surface coatings. Among these, the two most popular amino resins are urea-formaldehyde and melamine-formaldehyde. Amino resins are water-white, viscous materials that may contain added alcohols such as n-buta — nol, isobutanol, or isopropanol or sometimes their blends with aromatic hydrocarbons. Some water-soluble or water-reducible grades are also available that find application in waterborne coatings.
2.7.1 Chemistry of amino resins
|
|
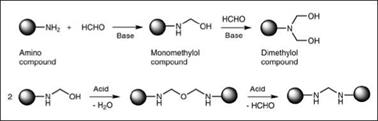
Some important compounds used in synthesis of amino resins are shown in Figure 2.22. Synthesis of amino resins for surface coatings typically involves two steps. In the first step, known as a methylo — lation reaction (Figure 2.23), the amino compound is reacted with formaldehyde to yield a methylolated intermediate. In the second step, known as an alkylation/etherification reaction (Figure 2.23), the methylolated intermediate is reacted with an alcohol to produce an alkyl ether of methylol groups. While methylol formation (step 1) is possible over the entire pH range, this reaction is generally carried out under basic conditions to obtain useful products.
|
Figure 2.23: Schematic representation of methylolation reaction |
Catalysis as well as type of amino compound have a significant influence on this stage; therefore, it is very important to mediate this stage for proper control of the molecular design of the final cross-linker. In the case of urea, under acidic conditions, polymerization of the methylol group is much faster than the methylolation reaction, which leads to insoluble products that are not suitable as cross-linking agents. Alkaline conditions favor methylol formation and provide improved stability against polymerization. Also important is the molar ratio of amino compound to formaldehyde. A molar excess of formaldehyde drives the reaction towards complete methylolation, while lower molar amounts of formaldehyde result in partially methylolated products. Melamine, with its six reactive sites, can yield a range of methylolated compounds depending upon the formaldehyde: melamine molar ratio.
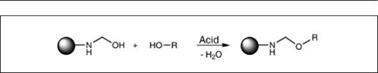
The methylol products formed by the above reaction are partially or fully etherified with alcohol to confer compatibility with co-resins and improved storage stability. This reaction is carried out under acidic pH conditions. The condensation reaction of two methylol groups (polymerization) competes with an alkylation reaction, which can be controlled by a molar excess of alcohol. The processing conditions are quite different for methylated aminoplasts compared to butylated ones, because for methanol, being water soluble, it is not possible to remove water of the reaction easily, while it is in the case of butanol. Therefore, to achieve the desired level of alkylation, a large excess of methanol, up to 1.6 mole per mole of the methylol group, is used. Primary alcohols react more readily with methylol groups than secondary alcohols, while tertiary alcohols are non-
|
Figure 2.24: Alkylation reaction |
reactive. Also, in general, reactivity of a primary alcohol decreases as the length of the aliphatic alcohol chain increases. The type of alcohol also influences viscosity of the resin for a given solid; for example, a butoxyl methyl melamine resin is less viscous than a corresponding methoxy methyl melamine resin.
 20 сентября, 2015
20 сентября, 2015  Pokraskin
Pokraskin 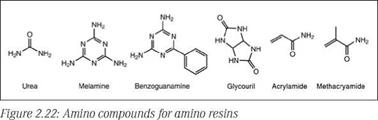
 Опубликовано в рубрике
Опубликовано в рубрике 