Polypropylene is one of the lightest engineering thermoplastics (SG = 0.90) and it has excellent moisture resistance. One of the major disadvantages of polypropylene is its poor impact strength at low temperatures. It does, however, offer excellent fatigue resistance and it is widely used for luggage, packaging, toys and storage battery cases.
![]()
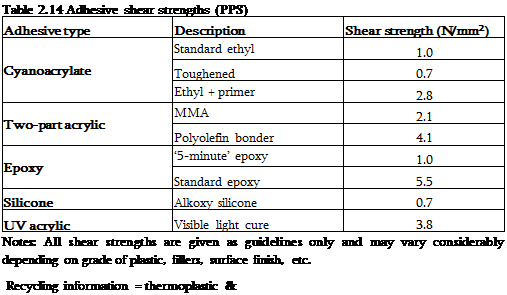 |
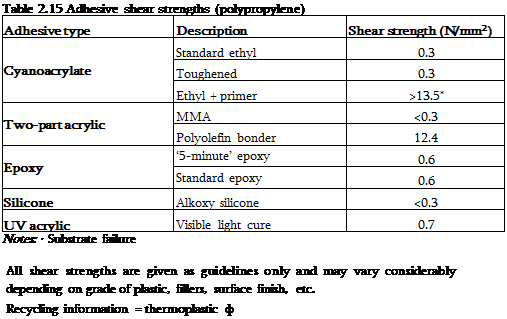 |
Unfilled PP is flammable and degraded by UV light; however, flame retardant and UV-stabilised grades are available.
Polypropylene is used in a very wide range of applications from pipes for chemical plant to automotive cooling fans and tool handles.
PP has a very low surface energy (29 mN/m) and so adhesives do not readily wet the surface. In addition, polypropylene is a very non-polar polymer consisting entirely of carbon and hydrogen atoms, whereas most adhesives contain oxygen, nitrogen and other electron-rich atoms and are polar materials. The carbon and hydrogen atoms in polyolefins are very unreactive towards many chemicals, thus precluding adhesion through chemical reactions. Polypropylene, therefore, generally requires pre-treatment prior to bonding although the polyolefin bonder did give excellent strengths in these trials [2] (Table 2.15).
 4 октября, 2015
4 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 