Physico-Chemical Evaluation of Stones to Be Adhered and Adhesive Mortars
The mineralogical composition, according to the Rietveld method, and the measured mechanical strength of stones to be adhered are presented in Table 2. According to these results, the Aktites stone samples (D1, D3) are hard and compact micritic dolomitic limestones, with a low to medium porosity, small grain size and high values of mechanical strength. The Mounichea stone samples are marly limestone/dolomitic limestone with an oolitic texture, brownish or yellowish to light grey-color, with a low to medium porosity and low values of mechanical strength (D2, D4, K). The Aktites samples were characterized by higher compressive strength, compared to the Mounichea samples, as a result of their lower content of clay material.
From the results of the DTA-TG analysis, the total bound water (Htb) identified in the temperature range from 100 to 400 oC corresponds to the dehydration of CSH, CAH and the residual bound water (Aggelakopoulou 2011). The dehydration of Ca(OH)2 (CH) occurred between 480 to 500 oC, while the decomposition of CaCO3 (Cc) took place at a temperature higher than 600 oC.
In particular, Figure 2 depicts the DTA curves for the NHL mortars without nano-titania at 1, 11 and 28 days of curing. The inset plot illustrates the evolution of the ratio unreacted-CH to formed-Cc at the same curing period for NHL mortars with and without nano-titania. As the setting process proceeds, mass losses attributed to the release of CO2 from CaCO3 increased,
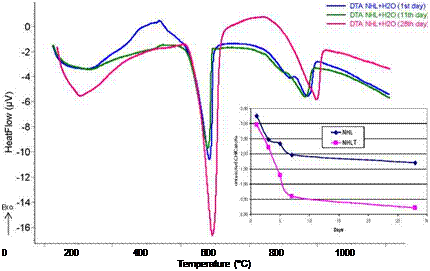 |
while the mass loss of CH dehydration decreased, due to the transformation of CH into hydraulic components and calcite. The lime consumption and the formation of hydraulic phases are more pronounced for the nano-titania mortars at different curing times. The same observation was also obtained from the thermal analyses of metakaolin-lime mortars with and without nano — titania.
|
Table 2. Mineralogical composition and compressive strength of the stones
|
Figure 2. DTA curves for NHL mortars without nano-titania cured for 1, 11 and 28 days along with the evolution of carbonation in NHL mortars without and with nano — titania (NHLT) illustrated in the inset plot.
Figure 3 depicts the results of the FTIR analyses after curing time of 1, 11 and 28 days. In the ML samples without nano-titania, portlandite was identified, exhibiting the characteristic absorption at 3640 cm-1. Furthermore, the absorptions at 990 and 540 cm-1, which are indicative of the neo-formed hydraulic components, are more pronounced in the samples with nano-titania, confirming enhancement of the hydration process.
SEM micrographs of the mortars ML1 without — (Figure 4a) and with nano-titania MLT1 (Figure 4b) after one year of curing corroborate the results of DTA analysis. SEM micrograph of MLT1 (Figure 4b) shows that a dense network of hydraulic amorphous components was formed, providing more elasticity to the mortar matrix. Moreover, characteristic hexagonal crystals of portlandite were located in both SEM micrographs; nevertheless in the case of MLT1 mortar, the portlandite crystals are obviously fewer. The FTIR spectra of the above studied mortars are in full accordance with the SEM micrographs, showing traces of portlandite and enhanced hydraulic compound formation after a one year curing (Maravelaki-Kalaitzaki 2013).
|
Figure 3. FTIR for metakaolin-lime mortars with (a) and without nano-titania (b) cured for 1, 11 and 28 days. Portlandite (P) still exists in the ML mortars after 28 days of curing. |
These variations can be explained by the TiO2 photocatalytic action for the mixtures with nano-titania; the latter leads both to calcite formation and enhanced hydration of CSH and CAH products, similar to what was observed during the early age hydration of Portland cement by adding increased dosage of fine titanium oxide (Jayapalan 2009).
|
Figure 4. SEM micrographs of (a) ML mortar and (b) MLT mortar showing portlandite crystals (P) and the hydraulic amorphous components. |
|
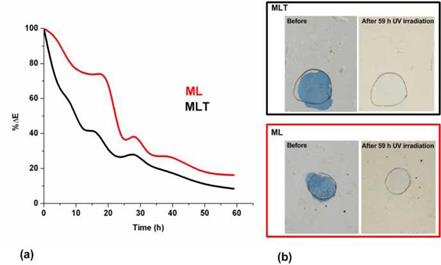
Figure 5. Decrease of ДЕ% of stains of methylene blue on mortars with metakalolin — lime and nano-titania (MLT) and metakaolin-lime (ML) after 59 h under UV irradiation. |
The photocatalytic properties of the nano-titania mortars were tested by applying both stains of methylene blue and methyl orange to the mortars’ surfaces and observing their discoloration. The intensive decrease of the total color difference ДЕ% observed on the mortar surface containing nano-titania stained with methylene blue after 59 h of UV irradiation, can be considered as an indication of the potential of nano-titania mortars to decompose organic pollutants (Figure 5).
 23 ноября, 2015
23 ноября, 2015  Pokraskin
Pokraskin 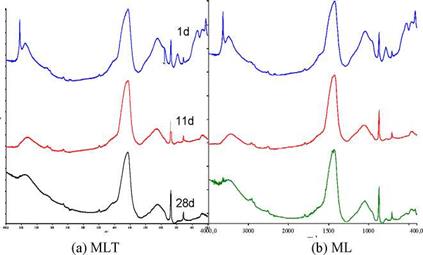

 Опубликовано в рубрике
Опубликовано в рубрике 