In the sulfate process, 2.4-3.5 t concentrated H2SO4 are used per tonne of TiO2 produced, depending on the raw material. During processing, some of this sulfuric acid is converted to sulfate, primarily iron(II) sulfate, the rest is obtained as free sulfuric acid (weak acid). Filtration of the hydrolyzate suspension can be carried out to give 70-95 wt.% of the SO42- in a weak acid fraction containing ca. 20-25% free acid, the remaining sulfate (5-30%) is highly diluted with wash-water.
In the past it was common practice to discharge acid directly into the open sea or coastal waters. For a long time the weak-acid problem was the subject of controversy. As a result the European Community decided to stop the discharge of weak acid into open waters by 1993.
The European TiO2 producers developed various effluent-treatment processes to meet the environmental requirements [2.50]. The most important processes are the precipitation of gypsum from the weak acid [2.51] and the concentration and recovery of the free and bound acid. Another outlet for the metal sulfate solution is the production of iron oxide pigments (see Section 3.1.1.2).
In the gypsum process, the acid effluent is treated in a first stage with finely divided CaCO3 to precipitate white gypsum. After filtering off, washing, and drying, the white gypsum is used for the manufacturing of plasterboard. In a second stage, the residual metal sulfates in the filtrate are precipitated as metal hydroxides and further gypsum by adding calcium hydroxide. This mixture, the so-called red gypsum, can be used for landfill. It has also been suggested to produce iron oxide pigments from the iron sulfate solution obtained after (partial) neutralization of the weak acid with CaCO3 or metallic iron [2.52].
In the recycling process, both the free and the bound sulfuric acid (as metal sulfates) can be recovered from the weak acid in the calcination furnace (Figure 2.4, k) and in metal sulfate calcination (Figure 2.5). The process consists of two stages:
1. Concentration and recovery of the free acid by evaporation
2. Thermal decomposition of the metal sulfates and production of sulfuric acid from the resulting sulfur dioxide.
|
g) Coal silo; h) Bunker; i) Mixing screw unit; j) Covered store for mixed filter cake; k) Calcination furnace; l) Waste-heat boiler; m) Cyclone; n) Electrostatic precipitator; o) Stirred tank; p) Storage tank; q) Pump; r) Cooler
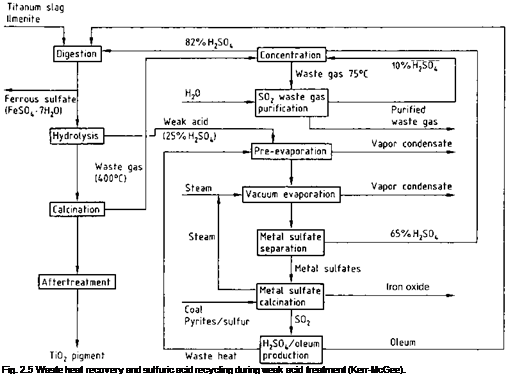
As a result of energy requirements only acid containing >20% H2SO4 can be economically recovered by evaporation. The weak acid is concentrated from ca. 2025% to ca. 28% with minimum heat (i. e., energy) consumption, e. g., by using waste heat from sulfuric acid produced by the contact process [2.53], or from the waste gases from the calcination kilns used in TiO2 production (Figure 2.5) [2.54].
Following preliminary evaporation, further concentration is carried out in multieffect vacuum evaporators. Since the water vapor pressure decreases strongly as the H2SO4 concentration increases, in general only two-stage evaporation can effectively exploit the water vapor as a heating medium. Evaporation produces a suspension of metal sulfates in 60-70% sulfuric acid (stage 1 in Figure 2.4). The suspension is cooled to 40-60 °C in a series of stirred tanks (stage 2, d) [2.55], giving a product with good filtering properties and an acid of suitable quality for recycling to the digestion process. Filtration (stage 3, e) is usually carried out with pressure filters [2.56] because they give a filter cake with an extremely low residual liquid content.
The concentration of the acid recycled to the digestion process depends on the quality of the titanium-containing raw material. For raw materials with a high titanium content, the 65-70% sulfuric acid separated from the metal sulfates must be further concentrated to give 80-87% acid (stage 5).
Concentration can be carried out in steam-heated vacuum evaporators, or by using the heat from the TiO2 calcination kilns [2.57]. Cooling the acid obtained after this concentration process yields a suspension of metal sulfates that can be directly used for digestion of the raw material. The metal sulfates recovered from the sulfuric acid in stage 3 contain sulfuric acid. They can be converted to a disposable material by reaction with calcium compounds [2.58]. Recently these metal sulfates (containing mainly ferrous sulfate) have found application as reducing agent for chromate in cement. Thermal decomposition of the metal sulfates to form the metal oxides, sulfur dioxide, water, and oxygen is energy intensive, but is advantageous from the ecological point of view. The energy requirement is ca. 4 x109 J per tonne offiltercake. Thermal decomposition is carried out at 850-1100 °C in a fluidized-bed furnace (stage 6). The energy is supplied by coal, pyrites, or sulfur. The sulfur dioxide produced by the thermal decomposition is purified by the usual methods, dried, and converted into sulfuric acid or oleum. This pure acid or oleum is mixed with the recovered sulfuric acid and used in the digestion process.
The metal oxides produced by thermal decomposition contain all the elements initially present in the raw material apart from the titanium, which has been converted into pigment. The mixture of metal oxides, mainly iron oxide, can be used as an additive in the construction materials or cement industry.
The continually increasing demand for environmentally friendly industrial processes has also led to the development of techniques for recycling the 5-30% sulfate remaining in the acidic wash water. [2.59]. In modern processes, up to 99% of the sulfuric acid can be recovered and reused in production.
The solid residue from the digestion reaction is most often disposed of after neutralization. A recently developed application for this material is its use as Ti source in blast furnaces for stabilizing the inner lining and increasing the life of the blast furnace [2.60].
In the chloride process, wastewater problems can arise, especially if the raw material contains <90% TiO2. The metal chloride by-products are sometimes disposed of in solution by the “deep-well injection” method (e. g., at Du Pont). The metal chloride solutions are pumped via deep boreholes into porous geological strata. Special geological formations are necessary to avoid contamination of the groundwater by impurities.
Increasing restrictions also apply to the chloride process, so that efforts are continually being made to use the iron chloride byproduct, e. g., in water treatment and as a flocculation agent. [2.61]. A process for treating metal chlorides with cement and alkaline compounds to produce rocklike aggregates for road building is described in [2.62]. Another option is to turn the iron chloride into iron oxide by means of the Ruthner process with recovery of hydrochloric acid [2.63]. At present many facilities using the chloride process have to neutralize their waste metal chlorides with subsequent disposal of the iron hydroxide obtained.
The recent development in the EU legislation concerning the necessity for reducing chromate in cement [2.32] might also have impact on the waste management from the chloride process. By turning the waste metal chlorides into FeSO4 with sulfuric acid (especially using spent acid) an FeSO4 reducing agent for cement can be obtained [2.64].
 26 сентября, 2015
26 сентября, 2015  Pokraskin
Pokraskin 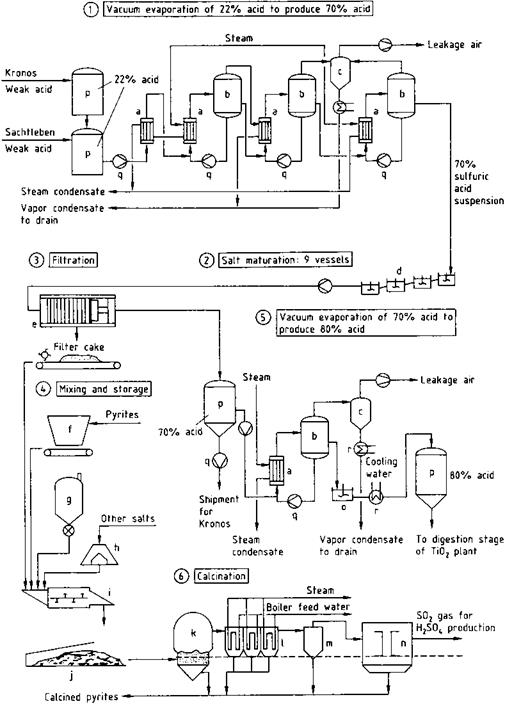
 Опубликовано в рубрике
Опубликовано в рубрике 