The sulfate method is summarized in Figure 2.2.
Grinding
The titanium-bearing raw materials are dried to a moisture content of < 0.1%. Drying is mainly intended to prevent heating and premature reaction on mixing with sulfuric acid. The raw materials are ground in ball mills to give a mean particle size of <40 pm.
Digestion
Batch digestion is usually employed. The ground raw materials (ilmenite, titanium slag, or mixtures of the two) are mixed with 80-98% H2SO4. It is possible to use 80% sulfuric acid and start the reaction by the addition of oleum. or the reaction can be started by adding water to a suspension of the raw materials in concentrated sulfuric acid. In either case the mixing enthalpy initiates the process, resulting in a vigorous digestion reaction with a maximum temperature of about 200 °C or even more.
The ratio of H2SO4 to raw material is chosen so that the weight ratio of free H2SO4 to TiO2 in the suspension produced by the hydrolysis is between 1.8 and 2.2 (the so-called “acid number”). The reaction in the digestion vessel (f) is started by adding water, dilute sulfuric acid, oleum, or sometimes steam. The temperature initially increases to 50-70 °C due to the heat of hydration of the acid. The exothermic sulfate formation then increases the temperature to 170-220 °C. If dilute acid or sparingly soluble raw materials are used, external heating is required.
After the maximum temperature has been reached, the reaction mixture must be left to mature for 1-12 h, depending on the raw material, so that the titanium — containing components become as soluble as possible. Digestion can be accelerated by blowing air through the mass while the temperature is increasing and also during the maturing period.
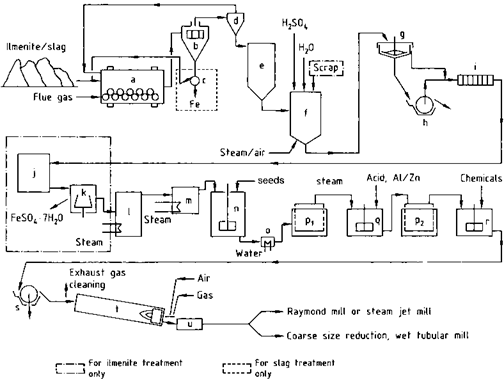
Several continuous digestion processes have been proposed [2.24]. A proven method is to continuously feed a mixture of ilmenite and water together with the acid into a double-paddle screw conveyor. After a relatively short dwell time (<1h), a crumbly cake is produced [2.25]. This process utilizes a more limited range of raw materials than the batch process because they need to be very reactive.
Due to the high cost of the titaniferous raw materials there have been numerous attempts to re-use the solid residue from digestion (with about 40-65% of TiO2) as raw material. Due to sophisticated and expensive equipment and/or processing conditions none of these developments have found large-scale realization [2.26]. Recently, however, there a redigestion process using the standard equipment and the standard processing technology has been found [2.27].
 19 сентября, 2015
19 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 