X-ray and infrared spectroscopy show that iron blue pigments have the formula MIFeIIFeIII(CN)6 • x H2O [3.171]. MI represents sodium, potassium or ammonium, ofwhich potassium and ammonium ions are preferred in industrial manufacture because they produce excellent hues; ammonium is the most common one.
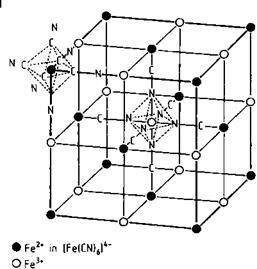
The crystal structure of the Fen FenI (CN)6 grouping is shown in Figure 3.19. A face — centered cubic lattice of Fen (ferrous ion) is interlocked with another face-centered cubic lattice of FenI (ferric ion), to give a cubic lattice with the corners occupied by ferrous ions. The CN ions are located at the edges of the cubes between each Fen ion and the neighboring Fem; the carbon atom of the cyanide is bonded to the Fen ion and the nitrogen atom is coordinatively bonded to the FeIII ion. The alkali-metal ions and water molecules are inside the cubes formed by the iron ions.
The presence of coordinative water is essential for stabilization of the crystal structure. Removal of this water, however carefully carried out, destroys the pigment properties. Many investigations helped to elucidate the structure of iron blue [3.1723.175].
Fig. 3.19 Crystal structure of iron blue [3.172].
 3.6.2
3.6.2
 25 ноября, 2015
25 ноября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 