1.4.1
Fundamental Aspects [1.60]
Many pigmented systems show typical color or structural changes when subjected to intense radiation or weathering [1.61]. The best known of these are yellowing [1.62], chalking, and loss of gloss. These processes involve photochemical reactions in which the pigment can act as a catalyst or in which the pigment itselfundergoes chemical changes.
Inorganic pigments are chemically very stable and are classed as one ofthe most stable coloring matters. This is especially true for oxide pigments, which often have a highly protective effect on the substrate [1.63]. Apart from the purely mechanical stabilization imparted by the pigment, this protective effect can give a coating an increased economic advantage. On the other hand, sulfide pigments can be oxidized by the atmosphere to form sulfates, which can be washed away by rain [1.64].
The photochemical processes that take place when TiO2-containing coatings undergo chalking have been elucidated [1.65]. The shorter wavelength radiation of sunlight acts on rainwater and atmospheric oxygen to form extremely reactive radicals (•OH, HO2^) that cause deterioration of the coating matrix by oxidative attack. Titanium dioxide pigments may be stabilized by reducing the number of radical — producing hydroxy groups on the surface of the TiO2 particles (e. g., by doping with zinc oxide). Alternatively, coatings of oxide hydrates are produced by aftertreatment of the pigment surface to give "reaction walls” on which the radicals are destroyed [1.66].
Continuous breakdown ofthe binder leads to the loss ofgloss. Surface gloss gives an attractive appearance and is usually desired, it is obtained by ensuring that the pigment particles are well dispersed. The stability ofgloss to weathering is therefore of great importance. If breakdown of the binder is so extensive that the pigment may be loosened, chalking takes place. Chalking is defined as loosening of pigment or
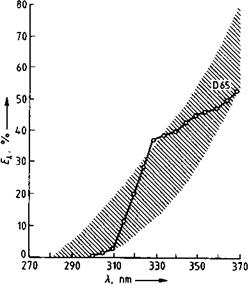
Fig. 1.12 Spectral energy distribution of daylight phase D 65 with permitted range of deviation (shading).
 extender particles following destruction of the binder at the surface. For standards, see Table 1.1 (“Coating materials”).
extender particles following destruction of the binder at the surface. For standards, see Table 1.1 (“Coating materials”).
Resistance to light and weather generally depend on the chemical composition, structure, defects, particle shape and size, and concentration of the pigment [1.67]. However, these properties also depend on the medium in which the pigment is used. Testing is carried out by open-air weathering, accelerated weathering, and chemical test methods.
Accelerated weathering is carried out in special weathering devices (e. g., Weath — erometer, Xenotest), which simulate exposure to sunlight and periods of rain. The spectral composition of the light used (Figure 1.12) should match the “global radiation” in temperate latitudes (global radiation denotes the sum of the direct solar radiation and indirect radiation from the sky). However, accelerated tests only allow limited conclusions to be drawn about light and weather resistances because it is not possible to accurately simulate all climatic conditions, their combinations and sequences, or sudden changes. Even so, natural weathering tests are not without problems: weather cannot be standardized, and a “uniform” weather therefore does not exist [1.68, 1.69]. Weathering results, even from successive years, can thus vary considerably [1.70]. Weather also varies with the geographical coordinates. For standards, see Table 1.1 (“Climates: Normalized”); the earth is classified into climatic regions and weathering adjustment scales exist [1.71].
None of the chemical methods (e. g., mandelic acid or methanol test) gives a perfect indication of weather resistance. Most of them only measure partial qualities of the photochemical reactivity [1.72].
The oxide pigments have the highest heat stability, followed by the sulfide pigments, which can even be used in enamels, and glass melts (e. g., cadmium pigments). The
oxide hydroxides and carbonates are less heat stable. Thermal stability is of great importance for modern coating techniques (e. g., for stoving finishes and other products requiring high temperatures). The heat resistance of a pigment primarily depends on the binder and the duration of heating. It is conveniently determined by observing hue changes in the pigmented coating system after a defined heat treatment, e. g., with white coatings yellowing occurs. For standards, see Table 1.1 (“PVC”). The chemical resistance of inorganic pigments, especially oxide pigments, is in general very high [1.73]. Other fastness properties such as overspray fastness, fastness to blooming, and fastness to plasticizers are of more relevance to organic pigments. For corrosion inhibiting (anticorrosive) pigments, see Section 5.2.
1.4.2
 1 сентября, 2015
1 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 