Black iron oxides obtained from the Laux process (see below) or other processes may be calcined in rotary kilns with an oxidizing atmosphere under countercurrent flow to produce a wide range of different red colors, depending on the starting material (Eq. (3.2)). The pigments are ground to the desired particle size in pendular mills, pin mills, or jet mills, depending on their hardness and intended use.
The calcination of yellow iron oxide produces pure red iron oxide pigments with a high tinting strength (Eq. (3.3)). Further processing is similar to that of calcined black pigments. High-quality pigments called copperas reds are obtained by the thermal decomposition of FeSO4 • 7 H2O in a multistage process (Eq. (3.1 a) and (3.1 b)), (Figure 3.1). If an alkaline-earth oxide or carbonate is included during calcination, the sulfate can be reduced with coal or carbon-containing compounds to produce sulfur
|
Fig. 3.1 Production of copperas red. a) dryer, b) rotary kiln (dewatering), c) rotary kiln, d) tank, e) thickener, f) filter. |
dioxide, which is oxidized with air to give sulfuric acid [3.8-3.11]. The waste gases and the dissolved impurities that are leached out in the final stage present ecological problems, however.
Lower quality products can be obtained by single-stage calcination of iron(II) sulfate heptahydrate in an oxidizing atmosphere. The pigments have a relatively poor tinting strength and a blue tinge. Decomposition of iron(II) chloride monohydrate in air at high temperatures (Eq. (3.4)) also yields a low-quality red iron oxide pigment [3.12]. In a new process, micaceous iron oxide is obtained in high yield by reacting iron(III) chloride and iron at 500-1000 °C in an oxidizing atmosphere in a tubular reactor [3.13].
Black Fe3O4 pigments with a high tinting strength can be prepared by calcining iron salts under reducing conditions [3.14]. This process is not used industrially because of the furnace gases produced.
Controlled oxidation of Fe3O4 at ca. 500 °C (Eq. (3.14)) produces a single-phase brown y-Fe2O3 with a neutral hue [3.15].
Calcination of a-FeOOH with small quantities of manganese compounds gives homogeneous brown pigments (Eq. (3.15)) with the composition (Fe, Mn)2O3 [3.16]. Calcination of iron and chromium compounds that decompose at elevated temperatures yields the corresponding pigments with the composition (Fe, Cr)2O3 [3.17].
 19 октября, 2015
19 октября, 2015  Pokraskin
Pokraskin 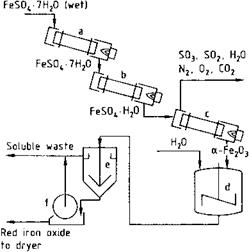
 Опубликовано в рубрике
Опубликовано в рубрике 