The red and black iron oxide pigments produced by the methods described have a Fe2O3 content of 92-96 wt.%. For special applications (e. g., ferrites) analytically pure pigments with Fe2O3 contents of99.5-99.8 wt.% are produced. The Fe2O3 content of yellow and orange pigments lies between 85 and 87 wt.% corresponding to FeOOH contents of 96-97 wt.%. For standards, see Table 1.1 (Iron oxide pigments: “Black, specification”; “Red, specification”; “Yellow, specification”; and “FeO content”). Variations of 1-2% in the iron oxide content are of no importance with respect to the quality of the pigments. Pigment quality is mainly determined by the quantity
|
Fig. 3.4 Transmission electron microscopic pictures (upper parts) and scanning electron microscopic pictures (lower parts) of iron oxide red of different particle size (bar = 0.5 ^m). Bayferrox® 110M shows a yellow tinge, while Bayferrox® 180M shows a bordeaux tinge. |
and nature of the water-soluble salts, the particle size distribution (hue and tinting strength are affected) and the average particle size of the ground product. The hue of red iron oxide is determined by the particle diameter, which is ca. 0.1 pm for red oxides with a yellow tinge and ca. 1.0 pm for violet hues (Figure 3.4).
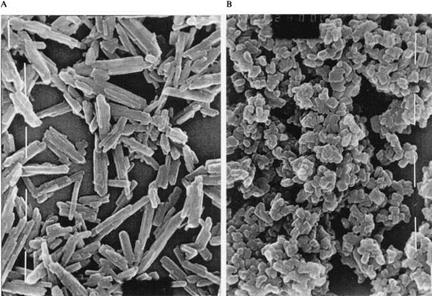
The optical properties of the yellow, usually needle-shaped, iron oxide pigments depend not only on the particle size, but also on the length to width ratio (e. g., length = 0.3-0.8 pm, diameter = 0.05-0.2 pm, length : diameter ratio = ca. 1.5-8). In applications for which needle-shaped particles are unsuitable, spheroidal pigments are available (Figure 3.5) [3.48]. Black iron oxide pigments (Fe3O4) have particle diameters of ca. 0.1-0.6 pm. An electron microscopic picture is given in Figure 5.7 in Section 5.1.6.
|
Fig. 3.5 SEM pictures ofa-FeOOH pigments. A) needle-like pigment (Bayferrox® 420) with standard silking, B) spheroidal pigment (Bayferrox® 915) with low silking effect. |
Some iron oxide pigments have a limited stability on heating. Red iron oxide is stable up to 1200 °C in air. In the presence of oxygen, black iron oxide changes into brown y-Fe2O3 at ca. 180 °C and then into red a-Fe2O3 above 350 °C. Yellow iron oxide decomposes above ca. 180 °C to form red a-Fe2O3 with liberation of water. This temperature limit can be increased to ca. 260 °C by stabilization with basic aluminum compounds. The thermal behavior of brown iron oxides produced by mixing depends on their composition.
3.1.1.5
 22 октября, 2015
22 октября, 2015  Pokraskin
Pokraskin 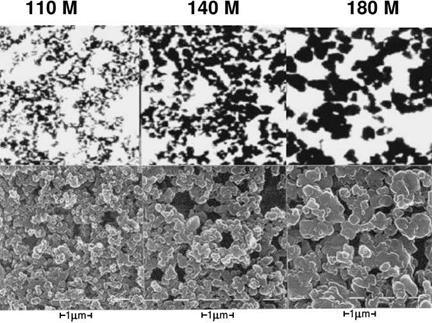
 Опубликовано в рубрике
Опубликовано в рубрике 