The basic ultramarine color is a rich, bright reddish blue, the red-green tone varying with chemical composition. The violet and pink derivatives have weaker, less saturated colors (see Figures 3.15-3.17 for reflectance spectra).
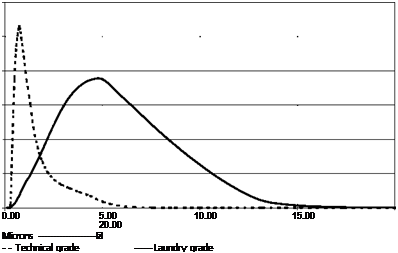
The color quality of commercial pigments is developed by grinding to reduce particle size and thus enhance tinting strength. Mean particle size ranges typically from 0.7 to 5.0 pm. Figure 3.18 shows the particle size distribution of a coarse grade, which is typically, used for laundry applications, compared to the fine grade which is more commonly used in technical applications such as inks and plastics. Although the fine pigments are lighter in shade and rather greener than coarser grades, when reduced with white, their color is brighter and more saturated.
 д 12 10
д 12 10
# 8
c
0
■s_
GO Q
4 2 0
Fig. 3.18 Particle size distribution for ultramarine blue.
With a refractive index close to 1.5, similar to that of paint and plastics media, ultramarine gives a transparent blue in gloss paints and clear plastics. Opacity is obtained by adding a small quantity of a white pigment. Increasing quantities of white give paler shades and a trace of ultramarine added to a white enhances the whiteness and acceptability.
In many applications ultramarine blue is stable to around 400 °C, violet to 280 °C and pink to 220 °C. All have excellent light fastness with a 7-8 rating (full and reduced shades) on the International Blue Wool Scale. Color fade attributed to light exposure or moderate heat is almost always caused by acid attack. Ultramarines react with all acids, and if there is sufficient acid, the pigment is completely decomposed, losing all color, to form silica, sodium and aluminum salts, sulfur, and hydrogen sulfide. Evolution of hydrogen sulfide with acids is a useful test for ultramarine.
Grades resistant to transient acidity are available, in which the pigment particles are protected by a coating of impervious silica. Blue and violet grades are stable in mildly alkaline conditions, but pink tends to revert to a violet shade.
Ultramarines are insoluble in water and organic solvents, so the color does not bleed or migrate from paints or polymers. This has led to ultramarines being approved for a wide range of food contact applications.
Being macromolecular, the fine ultramarine particles have a high surface energy and are cohesive. The finer grades, with their greater surface area, are therefore less easy to disperse than the coarser types, and some are available with pigment surfaces treated to reduce energy and improve dispersibility.
Ultramarines absorb moisture on the external particle surfaces and at the internal surfaces of the zeolite structure. External surface moisture (1-2% according to particle size) is driven off at 100-105 °C, but the additional 1% of internal moisture needs 235 °C for complete removal.
Ultramarine particles are hard and have been known to cause abrasion in equipment handling either dry or slurried pigment.
Specific gravity is 2.35, but the bulk density of the pigment powder is much lower, varying with particle size between 0.5 and 0.9 g cm-3.
Specific surface area varies with particle size and lies between 1 and 3 m2 g-1. Oil absorption also varies with particle size (usually 30-40 g%). The pH lies between 6 and 9. Ultramarine pigments are largely odorless, non-flammable, and do not support combustion.
3.5.3
 15 ноября, 2015
15 ноября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 