Hydrocarbon vapors may be almost quantitatively decomposed in a plasma into carbon and hydrogen [4.18]. This process can be used to make small-particle carbon blacks with new properties. However, an economical plasma-based commercial process is not yet known.
The Huels-electric-arc process was the only large-scale process using plasma reactions where large quantities of carbon black were produced as a by-product in the production of acetylene. However, this type of carbon black is no longer used as a pigment.
Since the price of both feedstocks and fuels is highly dependent on the petrochemical industry, several attempts have been made to find new raw materials. Processes for the manufacture of carbon black directly from coal [4.19] or for isolating carbon black from old tires have been studied. None of them, however, has achieved commercial importance up to now. On the other hand, clay, milled coal, and coke have found limited use as substitutes for very coarse carbon blacks, primarily thermal blacks and some SRF blacks. The increasing use of precipitated silicas in tires and mechanical rubber goods, mostly in combination with organo-silane coupling agents, which originally was indicative of an increasing search for new non-oil-based fillers, has led to new features of rubber properties.
4.4.7
Oxidative Aftertreatment of Carbon Black
Oxygen-containing functional groups on the surface of carbon blacks strongly influence their application properties (Figure 4.8). High contents of volatiles, i. e., high concentrations of surface oxides, decrease the vulcanization rate and improve the flow characteristics of inks. The gloss of coatings is increased, the color tone is shifted from brownish to bluish, and jetness often increases.
|
Fig. 4.8 Surface groups of pigment black. |
Some color blacks receive oxidative aftertreatment on a commercial scale to amend their color properties. Depending on the oxidizing agent and the reaction conditions selected, different types of surface oxides are formed in varying quantities.
The simplest method of oxidizing the carbon black surface is by aftertreating it with air at 350-700 °C. However, the degree of oxidation is limited. Higher contents of surface oxides and better process control are achieved with nitric acid [4.20], mixtures of NO2 and air [4.21], ozone, or sodium hypochlorite solutions [4.22] as oxidizing agents. As a rule, all strongly oxidizing agents may be used, either as a gas or in solution. Most surface oxidation of carbon black is carried out at elevated temperature.
Oxidized carbon blacks may contain up to 15 wt.% oxygen. They are strongly hydrophilic. Some of them form colloidal solutions spontaneously in water. In polar printing ink systems and coatings, a better wettability and dispersibility is achieved by surface oxidation, thus reducing binder consumption [4.23].
Surface oxidation of carbon black with nitric oxide and air can be carried out industrially in a fluidized-bed reactor [4.24]. A suitable aftertreatment unit consists of a preheating vessel, in which the carbon black is fluidized and heated, a reaction vessel to carry out the surface oxidation, and a desorption vessel, in which adsorbed nitric oxide is removed. Typical reaction temperatures are in the range 200-300 °C. Depending on the degree of oxidation, the residence time can amount to several hours. The nitric oxide acts primarily as a catalyst, the oxygen in the air being the genuine oxidizing agent.
Another common method of surface oxidation is carried out during pelletization. Nitric acid, instead of water, is used as a pelletizing agent. The surface is oxidized while the wet pellets are dried at elevated temperature [4.25]. Oxidation of powdery black with ozone is also carried out on a commercial scale.
4.5
 3 декабря, 2015
3 декабря, 2015  Pokraskin
Pokraskin 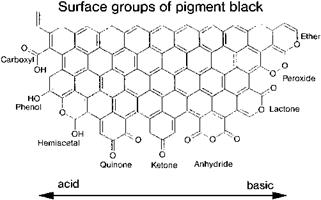
 Опубликовано в рубрике
Опубликовано в рубрике 