Anticorrosive pigments have been divided in Section 5.2.3 according to whether they act as active pigments, barrier pigments or sacrificial pigments. Micaceous iron oxide pigments have been one of the most popular barrier pigments for nearly a century and have been proven in intermediate and topcoats for long-term corrosion protection of structural steel work [5.100]. Probably the best-known example of the use of micaceous iron oxide (MIO) is the protection of the Eiffel Tower in Paris. MIO has been used in maintenance paints on this tower since its erection in 1889 [5.55]. MIO is a naturally occurring form of hematite (a-Fe2O3), which crystallizes as either granular or, more often, mainly lamellar and is also known as specular hematite. The term micaceous is used because of the more common lamellar particle shape of these pigments, but it does not contain mica (a special potassium aluminum silicate), the well-known filler used in coatings [5.55, 5.100]. MIO differs in particle size from the well-known red iron oxide color pigments (Figure 5.13).
MIO is dark gray in color with a metallic sheen. When viewed under an optical microscope by transmitted light, the thin-flake MIO particles appear as red translucent platelets and thicker and granular particles appear in a black shade [5.100]. The main function when using MIO in protective coatings is as a physical barrier, formed by the overlap of lamellar particles in the coating resulting in an elongation of the diffusion path for moisture and other corrosion stimulating substances through the coating (Figure 5.14) [5.100].
A comparative investigation of MIOs with different shapes, i. e. granular or a more lamellar shape (carried out within a study for the “Deutsche Bahn AG”) has shown no difference in the application ofeither grade in protective coatings with regard to the anticorrosive effect. This has been confirmed not only by accelerated laboratory tests but also by means of outdoor exposure in a coastal climate [5.103].
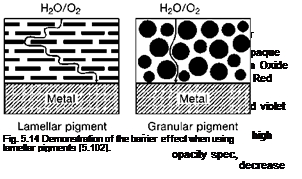 |
Fig. 5.13 Particle size and properties of iron oxide pigments based on a a-Fe2O3 [5.101].
This result leads to the conclusion, that there are other important product properties besides the particle shape that have an influence on the barrier effect. In this connection, it is discussed that effective MIO pigments should have a very high Fe2O3 content and very low water-soluble salt (i. e. chlorides and sulfates) content.
An ideal MIO pigment should not contain very fine particles in order to keep the wettable pigment surface small. High PVC in the coating can only be achieved when the oil absorption value of the MIO pigment is low [5.103]. In addition to the barrier mechanism, MIOs are recommended for use in topcoats as a protection against ultraviolet degradation [5.55].
MIO has also reached its importance as an anticorrosive pigment due to its properties such as very good thermal and chemical stability, good hardness (abrasion resistance) and good electrical resistivity [5.55]. The requirements and corresponding test methods on MIO-pigments are specified in ISO 10601 — Micaceous iron oxide pigments for paints — Specifications and test methods. ISO 10601 differentiates MIO — pigments into three grades depending on the lamellar particle content:
— Grade 1: thin-flake content >50%
— Grade 2: thin-flake content 10 to 50%
— Grade 3: thin-flake content <10% [5.104].
Synthetic MIO-pigments are also available, but these have not achieved the economic importance of the processed natural pigments (see Section 3.1.1). The currently available MIO-pigments on the market consist mostly of more lamellar particles. Other barrier pigments of a certain economical importance are aluminum flakes, steel flakes, phlogopite and muscovite mica.
5.2.14
 1 января, 2016
1 января, 2016  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 