Luminescent materials, also called phosphors, are mostly solid inorganic materials consisting of a host lattice, usually intentionally doped with impurities (Figure 5.43). The impurity concentrations generally are low, in view of the fact that at higher concentrations the efficiency of the luminescence process generally decreases (concentration quenching). Recently organic luminescent materials have gained considerable interest, in view of their application in organic light emitting diodes. In this section, however, only inorganic phosphors are discussed.
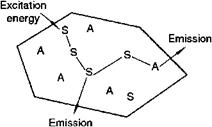 Fig. 5.43 Luminescent material, containing activator ions A (ions showing the desired emission) and sensitizing ions S (on which e. g. UV excitation can take place).
Fig. 5.43 Luminescent material, containing activator ions A (ions showing the desired emission) and sensitizing ions S (on which e. g. UV excitation can take place).
The absorption of energy, which is used to excite the luminescence, takes place by either the host lattice or by intentionally doped impurities. Moreover, the excitation energy can be transferred through the lattice by a process called energy transfer. In most cases, the emission takes place on the impurity ions. Quite frequently, the emission color can be adjusted by choosing the proper impurity ion, without changing the host lattice in which the impurity ions are incorporated.
5.5.3.1
 18 января, 2016
18 января, 2016  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 