Ion-exchange pigments are based on calcium ion exchanged silica gel. These anticorrosives are described as slightly porous pigments with a basic, calcium-exchanged silica surface, and a relatively high surface area. Therefore these pigments have a different chemical and physical identity compared to other types of anticorrosive pigments [5.92]. The typical properties of two commercially available ion-exchange pigments are shown in Table 5.11.
|
Tab. 5.11: Typical properties of ion-exchange pigments [5.93].
|
Figure 5.12 demonstrates the corrosion protection afforded by using calcium — exchanged silica [5.92].
It is reported in the literature that, when a corrosion-causing ion enters the film and comes into contact with the silica, an ion exchange occurs. The aggressive ion is locked onto the silica and the corresponding calcium ion migrates to the metal surface. The ion-exchange process should be continuous. Whenever an aggressive ion enters the paint film, the correspondingly released calcium ions form a protective layer at the metal coating interface with barrier properties, which prevents corrosion
|
|
Ca2+j
7777777777777777777777777777777
• Controlled release
• Maintains film integrity
Fig. 5.12 Corrosion protection by ion-exchanged pigments [5.92].
causing ions from contacting the metal surface [5.92]. As a result of the ion exchange process a neutralizing effect is also discussed [5.56].
Maybe, the most important real application of ion-exchange pigments is their use in coil coating primers, mostly in combination with other anticorrosive pigments.
5.2.10
 31 декабря, 2015
31 декабря, 2015  Pokraskin
Pokraskin 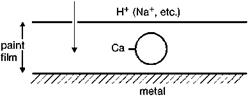
 Опубликовано в рубрике
Опубликовано в рубрике 