Corrosion leads to deterioration of metals by chemical or electrochemical reactions resulting from exposure to weathering, moisture, chemicals or other agents in the environment into which they are placed [5.50].
Often corrosion is described as an electrochemically driven process of energy exchange. Within this process, the metals, which were originally found in nature as ores, are reconverted back to their ores [5.51].
The required energy for the iron production from iron ore to cast iron and later to steel is enormous [5.52]:
172.6 kJ + CO2 + C ^ 2 CO 2 FeO + 2 CO ^ 2 Fe + 2 CO2 + 34.4 kJ
138.2 kJ + 2 FeO + C ^ 2 Fe + CO2.
In accordance with the rule of Lenz, the same energy will be released when iron is reconverted back to its ore. So, iron is not a thermodynamically stable metal. Because of this property it is a non-stable metal affected by corrosion, corrosion being the natural tendency of metals to move to their most stable form, for example as the oxide, sulfide and so on [5.51, 5.52].
Metals differ in the way by which they can be obtained from the ore. So, each metal shows a different readiness to react with oxygen or other elements and to return to its lower energy state [5.51]. The more energy that is needed for the metal production, the more readily the energy is released in the form of corrosion. Figure 5.8 shows that the most active metals are those with ready release of energy, which means these metals are most susceptible to corrosion [5.51].
|
Fig. 5.8 Electromotive series of metals [5.51]. |
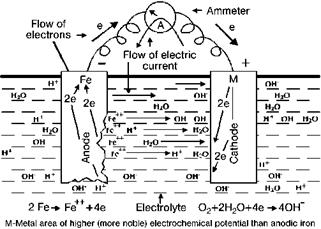
Any metal shows slight heterogeneities in its composition, for example from surface contamination or grain structure [5.51]. If such heterogeneous areas on a metal substrate come into contact with an electrolyte (an electrical conductor, such as moisture), these contiguous areas, having a slightly higher or lower tendency to corrode, will act as anodes and cathodes of an active electrochemical cell, as demonstrated in Figure 5.9 where iron is used as an example [5.51, 5.53].
|
Fig. 5.9 Electrochemical corrosion cell [5.51]. |
At the anode, the metal passes into solution as metal ions, which release electrons. Due to the presence of the electrolyte these electrons are able to migrate to the cathode and will be consumed by reactions with the environment [5.51]. Some electrochemical reactions are summarized in Figure 5.10.
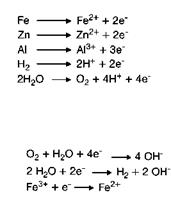 Anodic reactions * Examples
Anodic reactions * Examples
* Oxidation reactions
* Produce electrons
Cathodic reactions
* Examples
* Reduction reactions
* Consume electrons
Fig. 5.10 Electrochemical reactions.
As the main part of this section deals with anticorrosive pigments as such, we shall not go into more detail on the discussion of corrosion processes in this section. However, it should be mentioned that one key feature of the electrochemical process of corrosion is the presence of an electrolyte. This electrolyte is made up of rain, snow and dew, leading to ionogenic surface contamination. The electrolyte contains material coming from airborne pollution, such as dirt, water-soluble chloride and sulfate salts. Chlorides and sulfates are well known as so-called corrosion stimulators, which have a distinct influence on the course of the corrosion reactions [5.51, 5.54].
5.2.3
 23 декабря, 2015
23 декабря, 2015  Pokraskin
Pokraskin 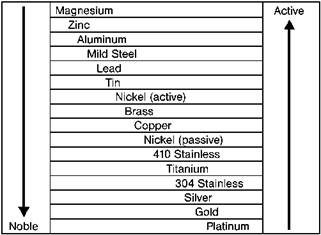
 Опубликовано в рубрике
Опубликовано в рубрике 