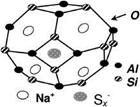
Reviews of work on the structure of ultramarine are given in Refs. [3.154-3.156]. Ultramarine is essentially a three-dimensional aluminosilicate lattice with entrapped sodium ions and ionic sulfur groups. A simplified structure is shown in Figure 3.14. The lattice has the sodalite structure with a cubic unit cell dimension of 9.10 A [3.157]. In synthetic ultramarine derived from china clay by calcination, the lattice distribution of silicon and aluminum ions is disordered. This contrasts with the ordered array in natural ultramarines.
In the simplest ultramarine structure, equal numbers of silicon and aluminum ions are present and the basic lattice unitisNa6Al6Si6O24 or (Na+)6(Al3+)6(Si4+)6(O2-)24 with a net ionic charge of zero as required for structural stability.
The nature of the sulfur groups responsible for the color and their incorporation into the sodalite structure is reviewed in Refs. [3.158-3.162]. There are two types of
Fig. 3.14 Simplified structure of ultramarine blue.
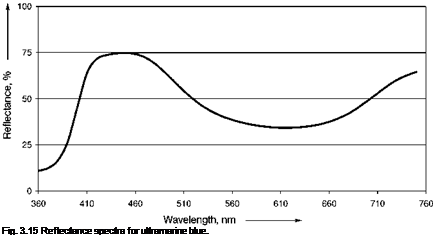 |
sulfur groups in blue ultramarine, S3- and S2-, both being free radicals stabilized by lattice entrapment. In the predominant S3- species, the spacing between the three sulfurs is 0.2 nm and the angle between them is 10 °. S3- absorbs a broad energy band in the visible green-yellow-orange region centered at 600 nm, whilst S2- absorb in the violet-ultraviolet region at 380 nm (Figure 3.15).
The basic lattice (Na+)6 (Al3+) 6 (Si4+)6 (O2-)
six of the silicon ions by aluminum. Every Al3+ must be accompanied by a Na+ so that the overall ionic charge for the structure is zero. Hence, six of the eight sodium sites are always filled by sodiums required for lattice stability and the remaining two sites are filled with sodiums associated with ionic sulfur groups. This means that only one S32- polysulfide ion can be inserted into the lattice (as Na2S3) even though subsequent oxidation to S3- leads to loss of one of the accompanying sodium ions. This gives a basic ultramarine lattice formula of Na7Al6Si6O24S3.
To increase the sulfur content and thereby improve color quality, the lattice aluminum content can be decreased by including high-silicon feldspar in the manufacturing recipe. This reduces the number of sodium ions needed for lattice stabilization and leaves more for sulfur group equivalence. A typical product would be Na6.9Al5.6Si6.4O24S4.2 with a stronger, redder shade of blue than the simpler type. An
alternative explanation for the improved color obtained by the inclusion of feldspar in the manufacturing recipe has been proposed [3.157].
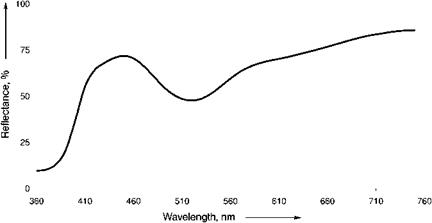
In violet (Figure 3.16) and pink (Figure 3.17) ultramarines, the lattice structure is little changed, but the sulfur chromophores are further oxidized, possibly to S3Cl-, S4, or S4-.
|
Fig. 3.16 Reflectance spectra for ultramarine violet. |
|
Fig. 3.17 Reflectance spectra for ultramarine pink. |
Ultramarines are zeolites, though lattice paths are restricted by 0.4 nm diameter channels. The sodium ions can be exchanged for other metal ions (e. g., silver, potassium, lithium, copper). The exchange with potassium ions can result in a slightly redder shade of ultramarine blue [3.163].
3.5.2
 14 ноября, 2015
14 ноября, 2015  Pokraskin
Pokraskin 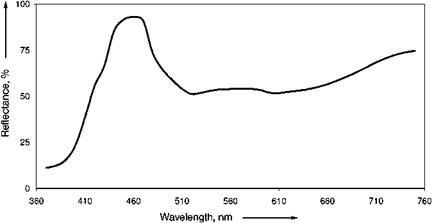
 Опубликовано в рубрике
Опубликовано в рубрике 