 |
This class of luminescent materials with more than one absorbed or emitted photon is gaining interest. Fundamentally, a well-selected combination of energy levels,
normally f-levels of lanthanide ions, is required to establish a cascade transition. With CaWO4:Yb3+,Er3+ as a first example, absorption of two photons with lower energy (e. g. deep red or IR), followed by emission of one photon with higher energy (visible) was proven by Auzel in 1966 [5.229]. Such so-called up-conversion processes are known for the summation of up to five photons. However, due to the comparatively low efficiency (< 1%) of the cascade process itself, as well as the low absorption crosssection of f-f-transitions, application is limited to areas where energy efficiency is not important.
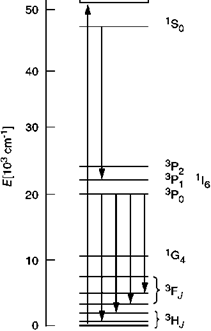
The opposite effect, the so-called down-conversion was reported in 1974, independently, for YF3:Pr3+ [5.230, 5.231]. Here, two photons with lower energy (visible) are emitted per photon absorbed at energy above about 5.7 eV (Figure 5.52). Absorption preferably takes place in the 5d band of Pr3+, as the underlying 4f-5d optical transition is parity allowed (Figure 5.53).
The emitted photons are located in the blue and red spectral region of the spectrum, respectively. In 1999, a first example of a quantum cutting material with two visible photons at similar wavelength (in the red spectral region), was presented: LiGdF4:Eu3+ (Figure 5.54) [5.232].
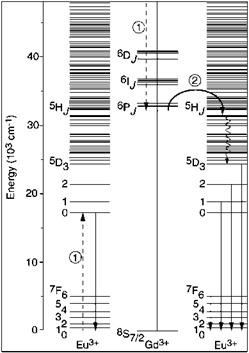 Fig. 5.54 Energy transfer and down — conversion in LiGdF4:Eu3+.
Fig. 5.54 Energy transfer and down — conversion in LiGdF4:Eu3+.
Such down-conversion phosphors are needed for energy efficient Hg-free lamps, which are driven by a Xe/Ne discharge. In order to establish f-f transitions with VUV-excitation and to avoid band-to-band absorption by the host lattice, large band-
gap materials such as fluorides have to be applied. Once again, new scientific input drives luminescence research.
5.5.7.2
 24 января, 2016
24 января, 2016  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 