The term “cadmium pigments” is understood in the pigment industry to refer to the pure sulfides and sulfoselenides as well as zinc-containing sulfides of cadmium. Cadmium pigments containing mercury were still used up to the middle of the last century but, because of their toxic properties, are no longer industrially significant. Cadmium sulfide occurs naturally as cadmium blende or greenockite and has a hexagonal wurtzite crystal structure. However, the mineral itself does not have any pigment properties. The color of cadmium pigments can be controlled via the composition (Table 3.5) and the size of the primary particles. The relative size of cadmium pigment particles is indicated in Figure 3.8.
|
Tab. 3.5: Color, chemical composition and Color Index number of cadmium pigments.
|
|
|
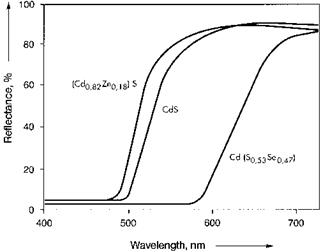
Cadmium pigments are semiconductors. Their color is determined by the distance between the valence and conduction bands in the crystal lattice. The high color purity of the pigments is due to the steep reflectance spectra (Figure 3.9), compare also Figure 3.10 in Section 3.3. They cover a wide color range of the visible light spectrum.
|
Fig. 3.9 Reflectance spectra of cadmium pigments [3.102]. |
3.2.1
 4 ноября, 2015
4 ноября, 2015  Pokraskin
Pokraskin 
 Опубликовано в рубрике
Опубликовано в рубрике 