The styryl dyes are neutral molecules containing a styryl group C6H5CH==C, usually in conjugation with an N, N-dialkylaminoaryl group. Styryl dyes were once a fairly important group of yellow dyes for a variety of substrates. They are synthesised by condensation of an active methylene compound, especially malo — nonitrile (9, X = Y = CN), with a carbonyl compound, especially an aldehyde. Styryl dyes have small molecular structures and are ideal for dyeing densely packed hydrophobic substrates such as polyester. C. I. Disperse Yellow31 (57; R = C4H9, R1 = C2H4Cl, R2 = H, X = CN, Y = COOC2H5) is a typical styryl dye.
|
|
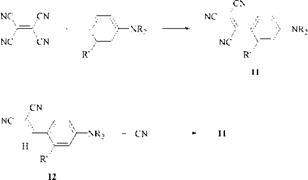
Yellow styryl dyes have now been largely superseded by superior dyes such as azopyridones, but there has been some interest in red and blue styryl dyes. The addition of a third cyano group to produce a tricyanovinyl group causes a large bathochromic shift: the resulting dyes (e. g., 11) are bright red rather than the greenish yellow of the dicyanovinyl dyes. These tricyanovinyl dyes have been patented by Mitsubishi for the transfer printing of polyester substrates. Synthetic routes to the dyes are the replacement of a cyano group in tetracyanoethylene
and the oxidative cyanation of a dicyanovinyl dye (12) with cyanide. The use of such toxic reagents is a hindrance to the commercialization of the tricyanovinyl dyes.
|
|
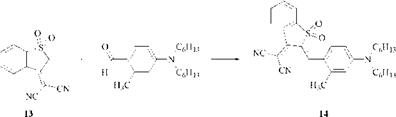
Blue styryl dyes are produced when an even more powerful electron-withdrawing group than tricyanovinyl is used. Thus, Sandoz discovered that the condensation of the sulfone (13) with an aldehyde gives the bright blue dye (14) for polyester. In addition to exceptional brightness, this dye also possesses high tinctorial strength (emax ca. 70 000). However, its lightfastness is only moderate.
|
|
 28 августа, 2015
28 августа, 2015  Pokraskin
Pokraskin 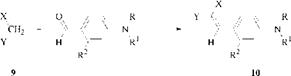
 Опубликовано в рубрике
Опубликовано в рубрике 