As a class, the dyes are bright and strong, but are generally deficient in lightfastness. Consequently, they are used in outlets where brightness and cost-effectiveness, rather than permanence, are paramount, for example, the coloration of paper. Many dyes of this class, especially derivatives of pyronines (xanthenes) are among the most fluorescent dyes known (see later).
|
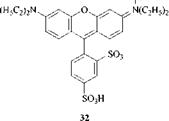
Typical dyes are the diphenylmethane Auramine O (28; R = CH3, X = C-NH 2; C. I. Basic Yellow 2); the triphenylmethane Malachite Green (27; R = CH3; R’ = H; C. I. Basic Green 4 ); the thiazine dye Methylene Blue (30; R = CH3; C. I. Basic Blue 9) used extensively in the Gram staining test for bacteria; the oxazine dye C. I. Basic Blue 3 (31; R = C2H5), and the xanthene dye C. I. Acid Red 52 (32). The bright magenta dye (32) is used in several ink-jet magenta inks, normally to improve the chroma (vividness).
The structural interrelationships of the diarylcarbenium dyes (26), triarylcar — benium dyes (27) and their heterocyclic derivatives are shown in Figure 2.6.
|
|
[1] J. Griffiths: Colour and Constitution of Organic Molecules, Academic Press, London, 1976, p. 250.
[2] J. Fabian, H. Hartmann: “Light Absorption of Organic Colorants”, Reactivity and Structure Conceptsin Organic Chemistry, vol. 12, Springer Verlag, Berlin, 1980, p. 137.
[3] H. Zollinger: Color Chemistry, 2nd ed., VCH Verlagsgesellschaft, Weinheim, 1991, p. 71.
[4] J. Griffiths, K. J. Pender, Dyes Pigm. 2 (1981) 37.
[5] C. Reichardt: Solvents and Solvent Effects in Organic Chemistry, 2nd ed., VCH Verlagsgesellschaft, Weinheim 1988, p. 107.
[6] Hoechst, DE 1 098 652, 1956.
[7] Hoechst, DE 1644 619, 1967.
[8] Dahl, DE 36 900, 1886.
[9] ICI, DE 508 499, 1929.
[10] Hoechst, DE 2 460 491, 1974 (M. Haehnke, F. Kohlhaas, F. Meininger, T. Papenfuhs).
[11] Hoechst, DE 2 344 443, 1973 (R. Neeb, T. Papenfuhs).
 29 августа, 2015
29 августа, 2015  Pokraskin
Pokraskin 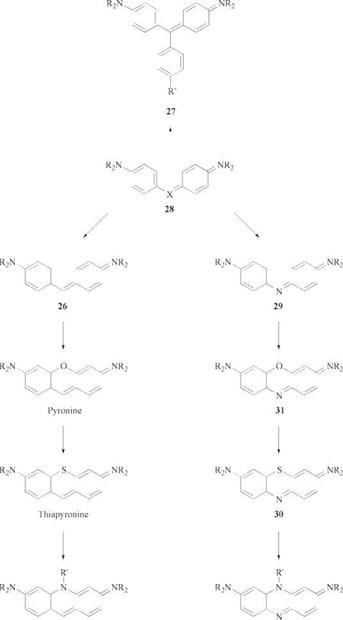
 Опубликовано в рубрике
Опубликовано в рубрике 