These dyes contain one or more carbonyl groups linked by a quinonoid system. They tend to be relatively large molecules built up from smaller units, typically anthraquinones. Since they are applied to the substrate (usually cellulose) by a vatting process, the polycyclic aromatic carbonyl dyes are often referred to as anthraquinonoid vat dyes.
In the vatting process the water-insoluble vat dye is reduced by sodium hydro — gensulfite (hydros) to the water-soluble phenate salt. This is less colored than the vat dye and has affinity for cotton. The cloth “dyed” with the phenate salt is placed in water, normally containing soap, and air is bubbled through the solution to regenerate the vat dye, which is trapped in the pores of the cotton (Scheme 2.4). The phenate salt may be stabilized by forming the sulfate ester. These stabilized, water-soluble salts of vat dyes, such as C. I. Solubilised Vat Blue 1 (5), are commercial products.
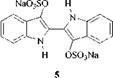 |
Scheme 2.4 The vatting process
Although the polycyclic aromatic carbonyl dyes cover the entire color range, only the blues, browns, greens, and blacks are important commercially. Typical dyes are the blue indanthrone (6), C. I. Vat Brown 3 (7), C. I. Vat Black 27 (8), and the C. I. Vat Green 1 (9), probably the best known of the polycyclic aromatic carbonyl dyes.
|
|
As a class, the polycyclic aromatic carbonyl dyes exhibit the highest degrees of lighfastness and wetfastness. The high lightfastness is undoubtedly associated with the absence of electron-donating and any electron-withdrawing groups other than carbonyl, so that the number of photochemically active sites in the molecule is restricted. The degree of aggregation is also a major contributory factor. The high wetfastness is a direct manifestation of the application process of the dyes.
[1] P. F. Gordon, P. Gregory, Organic Chemistry in Color, Springer-Verlag, Berlin, 1983.
[2] P. F. Gordon, P. Gregory, Organic Chemistry in Color, Springer-Verlag, Berlin, 1983, pp. 5-21.
[3] C. W. Greenhalgh, J. L. Carey, D. F. Newton, Dyes and Pigments 1 (1980) 103.
[4] British Patent 2,151,611 ICI 1985
 23 августа, 2015
23 августа, 2015  Pokraskin
Pokraskin 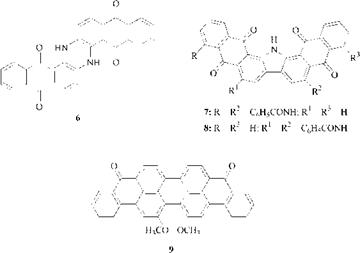
 Опубликовано в рубрике
Опубликовано в рубрике 