Like the hydroxy azo dyes, quinophthalone dyes can, in theory, exhibit tautomer- ism. Because the dyes are synthesised by the condensation of quinaldine derivatives (1) with phthalic anhydride, they are often depicted as structure (2), but this is incorrect, since the two single bonds prevent any conjugation between the two halves of the molecule.
|
|
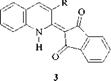
The dyes exist as structure (3), in which the donor pyrrole-type nitrogen atom is conjugated to the two acceptor carbonyl groups through an ethylenic bridge. In addition to the increased conjugation, structure (3) is stabilized further by the six-membered intramolecular hydrogen bond between the imino hydrogen atom and the carbonyl oxygen atom.
|
|
Quinophthalones provide important dyes for the coloration of plastics, e. g., C. I. Solvent Yellow 33 (3, R = H), and for the coloration of polyester. C. I. Disperse Yellow 54 (3, R = OH) is the leading yellow dye for the transfer printing of polyester.
 7 сентября, 2015
7 сентября, 2015  Pokraskin
Pokraskin 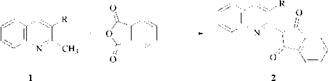
 Опубликовано в рубрике
Опубликовано в рубрике 