Most mono-anchor dyes are derivatives of cyanuric chloride (2,4,6-trichloro- 1,3,5-triazine [105-77-0]), a molecule of wide synthetic potential because the three chlorine atoms on the triazine ring differ in their reactivities [12]. The first chlorine atom exchanges with nucleophiles in water at 0 — 5 °C, the second at 35 — 40 °C, and the third at 80 — 85 °C. A wide variety of triazinyl dyes can thus be prepared by careful selection of the reaction conditions. Condensation of cyanuric chloride with a chromophore (“Chrom”) containing an amino group yields the highly reactive dichlorotriazinyl dyes 1 [11]. These very reactive dyes are sensitive to hydrolysis, and a suitable buffer is usually added to the powdered dye to increase its stability [13].
ci
When two of the chlorine atoms are substituted, for example with amino or alkoxyl groups, monochlorotriazinyl dyes 2 are obtained. These are considerably less reactive, and hence react with cellulose in the exhaust dyeing process only at relatively high temperature (80°C). Such dyes are especially advantageous for printing [14].
Cl
Chrom.—NH—N X = — NHR, -OR
N=<
X
2
The reactivity of monochlorotriazinyl dyes can be increased by replacing chlorine with fluorine (3), which allows the exhaust process to be carried out at 40°C [15].
|
3 |
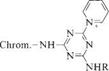
Reaction of monochlorotriazinyl dyes with tertiary amines also yields highly reactive dyes 4. One advantage conferred by the ammonium groups in these dyes is high water solubility [16].
|
4 |
The reactivity of dyes of this type is strongly influenced by the choice of tertiary amine. Nicotinic acid is preferred because it acts as a good leaving group and introduces comparatively little odor into the dyeing bath. The ammonium residue is also a better leaving group than, for example, chlorine, and allows the exhaust process to be conducted at 40 — 60 °C. In contrast to halogen-containing triazinyl dyes, these dyes do not require alkali in the application process, so dyeing can be conducted under neutral conditions.
In addition to the 1,3,5-triazines, other classes of heterocycles are important for the dyeing of cotton. Especially noteworthy are the halopyrimidine dyes 5 [17].
Cl Cl
Chrom.—NH— N
N=<
5
These dyes are less reactive than the triazines because the extra carbon atom reduces the ability of the ring to stabilize a negative charge. The reactivity of the system can be increased by introducing strongly electron withdrawing groups, including cyano [18], fluoro [19], or methylsulfonyl groups [20]. The pyrimidine ring can also be activated by inserting a carbonyl group between the chromo — phore and the heterocycle (6) [21].
|
|
|
|
|
|
|
|
 |
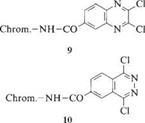

 Benzothiazole derivatives with good leaving groups at the 2-position of the heterocycle can also be used as reactive anchors. An example with chlorine as the leaving group is 11:
Benzothiazole derivatives with good leaving groups at the 2-position of the heterocycle can also be used as reactive anchors. An example with chlorine as the leaving group is 11:
The reactive group that has had the greatest impact on the market is the 2- sulfooxyethylsulfonyl group [9,24,25]. Treatment with alkali in this case causes the elimination of sulfuric acid to form a vinylsulfonyl moiety that reacts with cotton to give a dye — fiber bond [26]. Describing this as an elimination — addition sequence is not meant to rule out the possibility that the nucleophile attacks the p-carbon atom directly, without intervention of a vinyl intermediate [27]. Reactive vinylsulfones are also prepared from 2-chloroethylsulfonyl derivatives, which lead to the desired intermediates by elimination of hydrogen chloride:
Chrom.—SO,-CH,—CH2-X |+ Alkali
Chrom.-S02-CH=CH2 + HX
X = OSO, H, Cl
Numerous derivatives of ethylsulfonyl and vinylsulfonyl groups have been prepared in recent years, though none have approached the economic importance of the sulfate esters [28,29].
 10 сентября, 2015
10 сентября, 2015  Pokraskin
Pokraskin 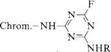
 Опубликовано в рубрике
Опубликовано в рубрике 