A long-term aim of dyes research has been to combine the brightness and fastness properties of anthraquinone dyes with the strength and economy of azo dyes. This aim is now being realized with heterocyclic azo dyes, which fall into two main groups: those derived from heterocyclic coupling components and those derived from heterocyclic diazo components.
All the heterocyclic coupling components that provide commercially important azo dyes contain only nitrogen as the heteroatom. They are indoles (85), pyrazolones (86), and especially pyridones (87); they provide yellow to orange dyes for various substrates.
|
|
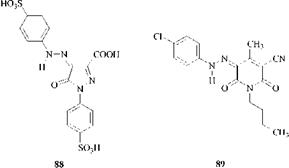
Many yellow dyes were of the azopyrazolone type, but these have now been largely superseded by azopyridone dyes. Azopyridone yellow dyes are brighter, stronger and generally have better fastness properties than azopyrazolone dyes. Both azopyrazolone and azopyridone dyes exist in the hydrazone tautomeric form. Typical dyes are the azopyrazolone C. I. Acid Yellow 23 (88) and the azopyridone (89).
|
|
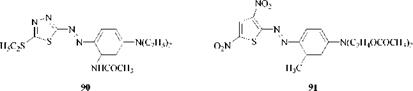
In contrast to the heterocyclic coupling components, virtually all the heterocyclic diazo components that provide commercially important azo dyes contain sulfur, either alone or in combination with nitrogen (the one notable exception is the triazole system). These S or S/N heterocylic azo dyes provide bright, strong shades that range from red through blue to green and therefore complement the yellow-orange colors of the nitrogen heterocyclic azo dyes in providing a complete coverage of the entire shade range. Two representative dyes are the thiadia- zole red (90) and a thiophene greenish blue (91). Both are disperse dyes for polyester, the second most important substrate after cellulose.
|
|
[1] Colour Index, Vol. 4, 3rd ed., The Society of Dyers and Colourists, Bradford, UK, 1971.
[2] P. F. Gordon, P. Gregory, Organic Chemistry in Colour, Springer-Verlag, Berlin, 1983, pp. 96-115.
[3] T. Zincke, H. Binderwald, ChemBer. 17(1884) 3026.
[4] P. F. Gordon, l3 Gregory, Organic Chemistry in Colour, Springer-Verlag, Berlin, 1983, pp. 104-108.
 22 августа, 2015
22 августа, 2015  Pokraskin
Pokraskin 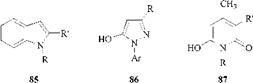
 Опубликовано в рубрике
Опубликовано в рубрике 