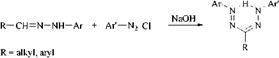 |
Coupling of Diazonium Compounds with Hydrazones. The most common method for the production of formazans is the coupling of diazonium salts with aryl hydrazones in an alkaline medium, possibly in presence of an organic solvent.
The industrially important 1-(2-hydroxyaryl)-3-aryl-5-(2-carboxyaryl)forma — zans are obtained by this process. The corresponding bis(2-hydroxy) compounds cannot be produced by this method because 2-hydroxyaryl hydrazines are very unstable compared to 2-carboxyaryl hydrazines.
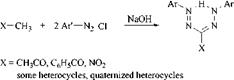 |
Double Coupling ofDiazonium Compounds. Some compounds with an activated methyl group can form symmetric formazans with strong diazo compounds by double coupling:
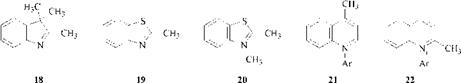 |
Examples of heterocycles with an activated methyl group are 18 [51], 19 [52], 20 [53], 21, and 22 [54].
During coupling of many methylene groups linked to two activating groups X and Y, the activating group X is exchanged for an azo group in the second coupling step. If the first coupling step is easier than the removal of the activating group X, then unsymmetrical formazans can be synthesized by coupling the initially formed hydrazone with another diazonium compound (Scheme 2.14).
![]() X-C-Y
X-C-Y
N-NH—Ar
|
X—CH,-Y
X = COOH, CONH2, CHO, СО-alkyl, СО-aryl, COCOOH, SOCH3 Y = X, COO-alkyl, CN, C=N-NH-aryl, S02-CH3, S02-aryl |
Scheme 2.14
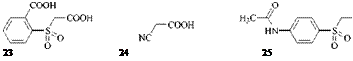 |
Only symmetrical formazans can be obtained, for example, from 23 [55], 24 [56], and 25 [57].
and from 4-oxopentanoic acid (levulinic acid), which couples to form the bis-for — mazan 26 [58]:
|
|
Unsymmetrical formazans can be prepared from 27 [56], 28 [59], 29 [60], and 30 [61] by stepwise coupling, preferably at different pH values.
|
|
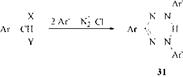
Doubly activated methane compounds with aryl substituents can also be converted to formazans 31 by elimination of both of the activating groups
|
X = COOH, CONH2, CHO, СО-alkyl, CO-aryl Y = X, CN, COO-alkyl |
Ester and cyano groups generally resist substitution by aryl azo groups and must first be hydrolyzed to produce carboxyl and carbonamide groups respectively, which can be more easily eliminated [62]. Unsymmetrical formazans can be produced according to Scheme 2.15.
|
Scheme 2.15 |
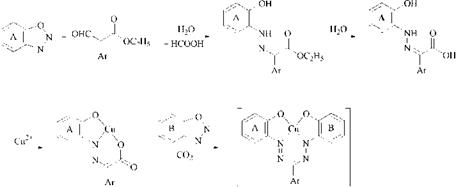
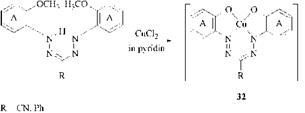
A special process for the production of coppered 1,5-bis(2-hydroxyphenyl)- formazans 32 is based on the demethylative copperization of 1,5-bis(2-methoxy- phenyl)formazans [63]. The method involves heating for a short time in pyridine, formamide, or dimethylformamide.
|
|
In an another process, the copper complexes are produced with alkylsulfonyl — amine as the complex-forming group [64].
Syntheses of formazans under phase-transfer conditions starting from hydra — zones or from compounds with active methylene groups are also possible [65].
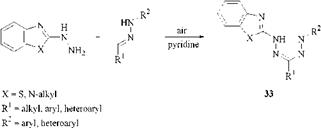
Oxidative Coupling of Azole Hydrazines with Hydrazones. Oxidative coupling of azole hydrazines with hydrazones takes place spontaneously in the presence of pyridine in air and is confined to the synthesis of heteroarylformazans, for example, 33 [66]:
|
|
Metallization. Bidentate formazans that are insoluble in water can be warmed with cobalt, nickel, and copper salts (preferably acetates) to form metal chelates in solvents such as methanol, ethanol, acetone, and dimethylformamide. Metal complexes of tri — and tetradentate formazans are much more stable. Metallization with divalent salts occurs rapidly at room temperature. On reaction with diazo — tized 2-aminophenols or 2-aminonaphthols, coupling and metallization with divalent metal salts can take place concurrently under the same conditions. When coupling is complete, the dye is usually fully metallized.
For production of Co™ 1:2 complexes, a cobalt salt is generally added after coupling and warmed to 60-90°C in a weakly alkaline medium. Reaction with chromium requires more severe reaction conditions, for example, refluxing for 20 h [67].
 5 сентября, 2015
5 сентября, 2015  Pokraskin
Pokraskin 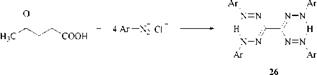
 Опубликовано в рубрике
Опубликовано в рубрике 