2.9.3.1 Introduction
Formazan dyes are closely related to azo dyes and are derived from the following basic structure:
12 3 4 5
-N=N-C=N-N- I H
(N1 me. so 1ST )
The position of the substituents is given according to IUPAC nomenclature using the prefixes 1-, 3-, and 5-. Formazans unsubstituted in the 1- and 5-positions and 1,5-dialkyl-substituted formazans are unknown. Aryl or heteroaryl groups are the most common 1,5-substituents. The 3- or meso position can be occupied by a variety of substituents (e. g., aryl, heteroaryl, H, OH, SR, halogen, NO2, CN, and alkyl).
The 1,5-diphenyl and 1,3,5-triphenyl derivatives were discovered almost simultaneously by H. von Pechmann [38] and E. Bamberger [39] in 1892. Interest in those compounds was renewed in 1939 when G. Lakon [40] and R. Kuhn and D. Jerchel [41] found that colorless tetrazolium salts were very sensitive to biological reduction processes and were converted to deeply colored formazans:
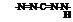
![]() H“
H“
In 1941 L. Hunter and L. B. Roberts produced metal complexes from forma — zan compounds [42].
R. Wizinger et al. introduced other complex-forming groups into formazans [43], which ultimately led to the discovery that metallized formazans could be used for textile dying.
Early reviews of formazans and their coordination compounds can be found in [44-50].
 5 сентября, 2015
5 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 