Since the mid-1980s considerable interest has been shown in strongly fixing reactive dyes, and the required high fixation values have increasingly been achieved with the aid of double anchors. Double-anchor dyes can be divided into two categories: those containing two equivalent reactive groups, and those with mixed-anchor systems.
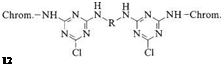 |
The first group includes structures in which two monochlorotriazine units are connected by a suitable bridge (e. g., 12). The synthetic approach to these compounds makes it possible to combine two different chromophores in a single system, opening the way to certain color shades that are not easily accessible via a single chromophore [30].
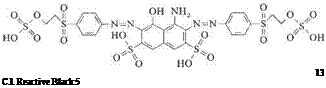 |
The category of reactive dyes with two equivalent anchors also encompasses products containing two vinylsulfonyl or 2-sulfooxyethylsulfonyl groups. An example is C. I. Reactive Black 5, 20505 [17095-24-8] (13), which not only displays a high degree of fixation but is also accessible by a synthetic route that is both simple and economical:
Selective modification of the reactive anchors makes it possible to vary the physical property profile of the dye.
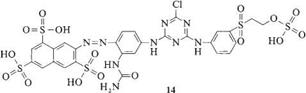
Mixed-anchor systems in reactive dyes were first described in 1959. Such “bifunctional” dyes were first marketed in the early 1980s. These products are characterized by two anchors with differing reactivities: a more reactive 2-sulfo — hydroxyethylsulfonyl group and a less reactive monochlorotriazinyl residue. An
example of such a system is 14:
 |
Warm — and cold-dyeing double-anchor dyes are prepared by incorporating halo — triazinyl and vinylsulfonyl reactive anchors. The bond between the triazine ring and a fiber is stable under basic conditions, whereas that to the vinylsulfonyl group is stable to acid. A combination of the two anchor systems therefore produces a dye with
good fastness over a wide pH range. Relative to dyes based on dichlorotriazinyl, dichloroquinoxalinyl, or difluorochloropyrimidyl groups, mixed double-anchor dyes show greater acid fastness and more satisfactory behavior with respect to laundry water containing peroxide detergents. The dyes 15, introduced in 1988, include products in which a fluorotriazine unit bridges a vinylsulfonyl anchor and a chromophore [31]. Here the two reactive systems exhibit similar reactivities.
|
|
15
Other mixed reactive systems have also been patented in addition to the double-anchor dyes so far described, including combinations of monofluorotriazine with difluorochloropyrimidine [32], alkoxychlorotriazine [33], or 2-(sulfothio) ethylsulfonyl groups [34]. Other examples incorporate a difluorochloropyrimidine ring in conjunction with a vinylsulfonyl group [35].
Despite the growing importance of mixed reactive systems, only the mono- chloro-triazinyl/vinylsulfonyl and monofluorotriazinyl/vinylsulfonyl dyes described above have become commercially established.
 10 сентября, 2015
10 сентября, 2015  Pokraskin
Pokraskin 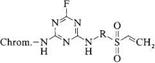
 Опубликовано в рубрике
Опубликовано в рубрике 