Depending on the basicity and solubility of the amines being diazotized, the following diazotization methods find industrial use:
a) Direct Diazotization. The primary aromatic amine is dissolved or suspended in aqueous hydrochloric or sulfuric acid, and a concentrated aqueous sodium nitrite solution is added. An excess of 2.5-3 equivalents of acid per equivalent of amine is used. A temperature of 0-5 °C is maintained by adding ice.
b) Indirect Diazotization. Amines with sulfonic or carboxylic acid groups are often difficult to dissolve in dilute acid. Therefore, the amine is dissolved in water or a weak alkali, and the calculated amount of sodium nitrite solution is added to this amine solution which is stirred into the ice-cooled acid solution already in the vessel. The acid can also be added to the amine-nitrite mixture already at hand.
c) Diazotization of Weakly Basic Amines. Weakly basic amines are dissolved in concentrated sulfuric acid and diazotized with nitrosylsulfuric acid, which is easily prepared from solid sodium nitrite and concentrated sulfuric acid.
d) Diazotization in Organic Solvents. The water-insoluble or sparingly soluble amine is dissolved in glacial acetic acid or other organic solvents and, where necessary, diluted with water. After the addition of acid it is diazotized in the usual manner with sodium nitrite solution. Nitrosylsulfuric acid, nitrosyl chloride, alkyl nitrites, or nitrous gases can also be used instead of sodium nitrite. Temperature, pH, and the concentration of the diazotizing solution often have a considerable effect on the progress of diazotization. Physical properties (distribution, particle size) and the addition of emulsifiers and dispersing agents influence the diazotization of slightly soluble amines.
Certain aromatic amines require special diazotizing processes: 1-Aminonaphthols , such as 1-amino-2-naphthol and several 1-aminonaphthol- sulfonic acids, such as 1-amino-2-naphthol-4-sulfonic acid, are oxidized to the respective quinone by nitrous acid. Diazotization can, however, take place under normal conditions in the presence of catalytic quantities of metal salts, such as copper or zinc salts.
o-Diamines, such as 1,2-phenylenediamine and 1,8-naphthylenediamine, undergo cyclization during the normal diazotization process to form triazoles:
|
|
The desired bis-diazotization with o-, m and p-phenylenediamine is achieved in glacial acetic acid with nitrosylsulfuric acid. 1,8-Naphthylenediamine can be bis-diazotized in an excess of acid.
Diazonium compounds are generally stable only in aqueous solution at low temperatures. When heated, they frequently decompose by eliminating nitrogen to form the corresponding phenol. Some amines, however, can be diazotized at temperatures up to 40 °C. Metal ions also accelerate the decomposition of diazonium compounds. Therefore, diazotization is usually carried out in wooden vats or iron stirring vessels with an acid-proof lining or rubber coating.
 20 августа, 2015
20 августа, 2015  Pokraskin
Pokraskin 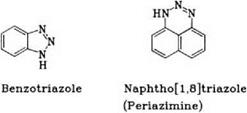
 Опубликовано в рубрике
Опубликовано в рубрике 