Di- and triarylcarbenium dyes [1] belong to the class of polymethine dyes and can be considered as branched polymethines. The branches are created by two aryl rings, in which the polymethine chain is incorporated, and by another R group bonded to the central (meso) methine carbon atom (1).
|
|
The R group possesses я-electrons or lone pairs of electrons that can interact with the rest of the я-electron system. The most important electron donor is the amino group. Triarylmethine dyes are usually divided into mono-, di-, and triami — notriarylmethine dyes. In some di — and triarylmethine dyes, the ring carbon atoms ortho to the central methine carbon atom are bonded via a heteroatom to form a heterocyclic six-membered ring. These include the acridine, xanthene, and thiox — anthene dyes.
 28 августа, 2015
28 августа, 2015  Pokraskin
Pokraskin 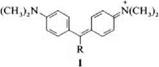
 Опубликовано в рубрике
Опубликовано в рубрике 