The diphenylmethyl and triphenylmethyl cations can be considered the basic chromophores of di — and triarylmethane dyes [1-4]. However, the electronic state of the donor-substituted di — and triarylmethine dyes can be described better by breaking down the chromophore of these dyes into a straight — or branched-chain polymethine subchromophore, respectively, and two ethylene units (from the formal cleavage of the benzene rings) [2]. This model allows the similarities between the di — and triarylmethine dyes and the polymethines to be recognized.
The introduction of two dimethylamino groups into the diphenylmethyl cation gives Michler’s hydrol blue (1, R = H). This compound shows a strong bathochro — mic shift and an increase in intensity of the absorption band at the longest wavelength relative to the diphenylmethyl cation [ 1, R = H; Xmax (log e) = 607.5 nm (5.17)]. Donor substituents R in diarylmethane dye 1 cause a blue shift of the longest-wavelength absorption band, whereas acceptor groups generally cause a red shift [2].
|
|
Malachite green (2, R = H) has two absorption bands in the visible spectrum at hmax (log e) = 621 (5.02) and 427.5 nm (4.30). The intense absorption band at longest wavelength (x band) is shifted to the red compared with Michler’s hydrol blue. The second band (y band) is in the shorter-wavelength part of the spectrum and is significantly weaker [1-3].
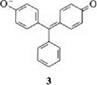 |
Substituents in the meta and para positions of the meso-phenyl ring shift the position of the absorption band at longest wavelength. If a dimethylamino group is introduced into the para position of the meso -phenyl ring, crystal violet is obtained [ 2, R = N(CH3)2], instead of malachite green. The absorption spectrum of the former has a hypsochromically shifted x [~Xmax (log e) = 589 nm (5.06)] and no у band [9]. The hydroxytriarylmethane dyes, which are isoelectronic with cationic triarylmethane dyes, absorb at a shorter wavelength than the latter. The longest-wavelength absorption band of benzaurin (3) is at kmax = 585 nm compared with Xmax =621 nm in malachite green [2].
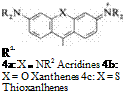 |
By bonding two phenyl rings via a heteroatom bridge in the 2- and 2′-positions, the yellow acridine dyes (4a), the red to violet xanthene dyes (4b), or similarly colored thioxanthene dyes (4c) in the case of amino-substituted di — or triarylmethane dyes are obtained. Because of their rigid molecular skeletons these compounds fluoresce. Hydroxyxanthenes behave similarly (e. g., fluorescein) [2].
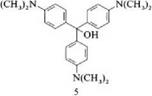 |
On protonation of crystal violet, the color changes first to green and then to yellow. The green coloration is caused by protonation of one of the dimethylamino groups and can be explained by the appearance of the y band. Protonation of another amino group changes the color to yellow. Amino groups whose conjugation with the ring system is reduced for steric reasons are readily protonated so the intensity of the absorption bands is lowered in comparison with systems having unhindered conjugation because of the proportion of protonated species. In an alkaline medium, decoloration occurs because of the formation of triphenyl — methanol (e. g.,5) [2].
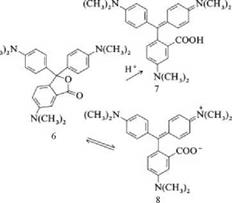
Color formers are very sensitive to the addition of acids and solvents. In acid, crystal violet lactone (6) is only partially converted to the colored carboxylic acid (7) with opening of the lactone ring [Xmax (log e) = 603 nm (4.41) in methanol / acetic acid (1/1)].
|
|
The acid is in equilibrium with colorless lactones protonated on the nitrogen atom. In highly polar solvents such as trifluoroethanol, hexafluoro-2-propanol, or phenol, the lactone ring opens to give the zwitterion (8). The longest-wavelength band of (8) [max (log є) = 593 nm (4.91) in hexafluoro-2-propanol] is shifted hypsochromically relative to that of (7). As in the case of rhodamine dyes [5], this reaction is a solvent-dependent intramolecular Lewis acid-base equilibrium. The high intensity of the band shows that the equilibrium is shifted strongly to the zwitterionic compound.
 29 августа, 2015
29 августа, 2015  Pokraskin
Pokraskin 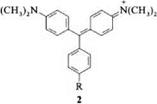
 Опубликовано в рубрике
Опубликовано в рубрике 