This chapter gives a short survey on cationic dyes and shows typical representatives of each class of cationic dyes. In general, the dyes are classified according to their chemical structure.
2.4.3.1 Dyes with Delocalized Charge
Cationic dyes with delocalized charge are classified with the methine dyes (see Section 2.6). They may be viewed as vinylogous amidinium salts 3 [1]:
|
|
All of these dyes can be represented by various resonance formulas. They are characterized by high color strength.
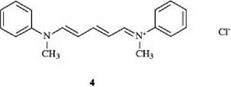
Dyes in which both charged terminal atoms are not part of a heterocyclic ring are called streptocyanine dyes. An example is 4, which dyes paper in a greenish yellow shade [2].
|
|
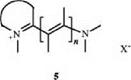
In the hemicyanine dyes, one of the charged terminal atoms is part of a heterocyclic ring, and the other is a nitrogen atom directly linked to the methine chain (5).
|
|
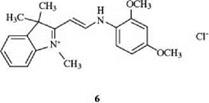
An example of this type is the enamine dye 6, which confers a yellow color to polyacrylonitrile fibers [3].
|
|
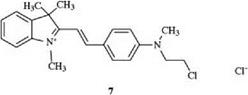
Insertion of an aryl residue between the second nitrogen atom and the methine chain leads to the styryl dyes, which dye polyacrylonitrile in red and purple shades, e. g., 7 [4].
|
|
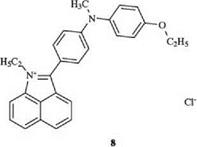
In a special form of this type, the chain may be completely missing. This leads to zeromethine dyes such as 8 [5], which dye polyacrylonitrile in very lightfast violet and blue shades.
|
|
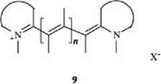
If the second charged terminal atom is also part of a heterocyclic ring, the cyanine dyes are obtained (9).
|
|
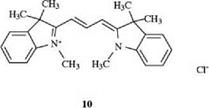
These dyes are used predominantly as photographic spectral sensitizers, but some years ago a new application in optical data storage media was found. Compound 10 dyes paper in brilliant pink shades [6]:
|
|
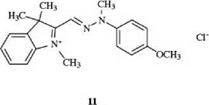
One or several of the carbon atoms in the chain may be replaced by nitrogen, as is indicated by the prefix “aza.” Depending on the number of nitrogen atoms introduced into the chain, mono-or diazadimethine dyes are obtained. Tri- and tetraazamethine dyes are also known. An example is dye 11, which gives polyacrylonitrile fibers a golden yellow color [7].
|
|
Another example is the above-mentioned dye 2, which dyes polyacrylonitrile in lightfast blue shades [8].
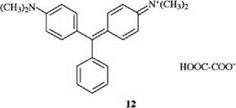
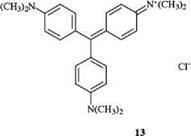
Diphenylmethane and triphenylmethane dyes are monomethine dyes with two or three terminal aryl groups, of which at least one, but preferably two or three, are substituted by a donor group para to the methine carbon atom. The most important donor is the amino group. Important dyes belonging to this class include the well-known malachite green (12) [9] and crystal violet (13) [10], which are some of the oldest synthetic cationic dyes:
|
|
|
|
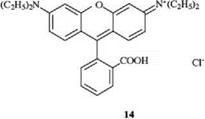
Introduction of an oxygen bridge into the triphenylmethane dye molecule leads to the xanthene dyes. The color is shifted from blue to red. The restricted rotation of the phenyl groups inhibits radiationless de-excitation and gives rise to very strong fluorescence. Rhodamine B (14) is used for dyeing paper [11] and as a laser dye.
|
|
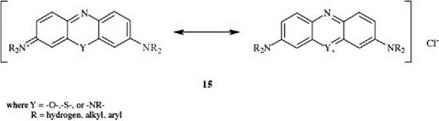
Azine dyes, which have the general formula 15, are ring-closed azadiphenyl — methane or indamine dyes. Examples are phenazine dyes, oxazine dyes, and thia — zine dyes.
|
|
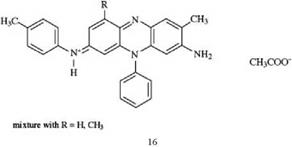
The phenazine dye mauveine (16), discovered by perkin [12], was the first synthetic dye produced on an industrial scale.
|
|
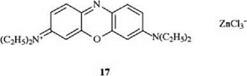
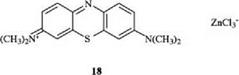
Of current importance are the oxazine dye 17 [13] and the thiazine dye 18 (methylene blue) [14].
|
|
|
|
 26 августа, 2015
26 августа, 2015  Pokraskin
Pokraskin 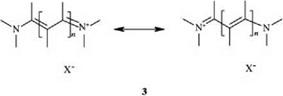
 Опубликовано в рубрике
Опубликовано в рубрике 