In general, metallization is accompanied by the lost of protons, and therefore it can be monitored potentiometrically, similar to a neutralization reaction. With the exception of 1:1 chromium complex formation, all metallization reactions are promoted by acid-binding agents.
CopperComplexes. The preparation of copper and nickel complexes of tridentate metallizable azo and azomethine dyes is easily carried out in aqueous media with copper and nickel salts at pH 4-7 in the presence of buffering agents such as sodium acetate or amines. Sparingly water soluble precursors can be metallized in alkaline medium at up to pH 10 by using an alkali-soluble copper tetram(m)ine solution as coppering reagent, which is available by treating copper sulfate or chloride with an excess of ammonia or alkanolamines [3].
Three other approaches to copper complexes are also applicable, all of which do not start from o, o’-dihydroxyazo compounds. These are valuable and convenient methods in those cases where o, o’-dihydroxyazo compounds are difficult to prepare from diazotized o-aminophenols. The first method is the simultaneous dealkylation and coppering of o-alkoxy — o’-hydroxyazo dyes. The dealkylative reaction is assisted by the coordination of the alkoxyl oxygen atom to the copper ion and is carried out by heating above 80 °C with copper tetrammonium sulfate in presence of an alkanolamine or pyridine [4]. The 1:2 opper complex 1, in which the the ether groups are uncoordinated, reacts only very slowly to give the copper chelate of the corresponding o, o’-dihydroxyazo dye [5].
|
|
A further coppering method is oxidative coppering [6], which involves treatment of an o-hydroxyazo dye with an oxidizing agent in the presence of cop — per(n) ions. The most widely used oxidizing agent is hydrogen peroxide, but Na2O2, peroxy acids, Na2S2O8, and NaBO3 have also been employed. The conditions must be mild, in general in aqueous medium at 40-70°C and pH 4.5-7.0, because more drastic treatment degrades the original dye. The third method starts from o-chloro- o’-hydroxyazo compounds and gives the desired copper complexes on treatment with a copper salt in alkaline medium at 50°C [7] (Scheme 2.9).
|
Scheme 2.9 Example for an oxidative coppering reaction |
This nucleophilic replacement of halogen atoms proceeds under mild conditions due to the neighboring azo group and the presence of copper ions. The o — sulfonic acid group is also susceptible to the copper-mediated nucleophilic substitution, and other nucleophiles, such as alkoxy, alkylamino, cyano, and sulfinic acid can also replace the halogen atom in the position ortho to the azo group in the presence of copper ions.
Dealkylative and oxidative coppering and subsequent demetallation in aqueous mineral acids are technical processes to prepare o, o’-dihydroxyazo compounds, which are not available from o-aminophenols by diazotation and coupling reactions.
Chromium Complexes. Because of their high stability chromium complexes of tridentate azo dyes are the most important class of metal-complex dyes. This is due to the reluctance of hexacoordinated chromium(m) complexes to exchange ligands, which, however, complicates the preparation of chromium complex dyes from hexaaqua chromium(m) salts, and makes it possible to prepare triaqua 1:1 chromium complex dyes. Generally, 1:1 chromium complexes can be made in
acid medium below pH 4, whereas 1:2 chromium complexes are prepared at higher pH in weakly acid to alkaline medium. The stability of the chrome dyes parallels the pH conditions for production. The 1:1 chromium complex dyes are only stable in the presence of mineral acids, and in slightly alkaline medium they revert to the metal-free dye and chromium hydroxide. The 1:1 chromium complexes based on o-carboxy-o’-hydroxyazo dyes are much less stable than those based on o, o’-dihydroxyazo dyes. For example, the 1:1 chromium complex of 1-phenyl-3-methyl-4-(2′-carboxy-5′-sulfophenylazo)pyrazol-5-one decomposes slowly already at room temperature and under neutral conditions. On the other hand, 1:2 chromium complex dyes are unstable at high acidity and disproportionate into the corresponding 1:1 chromium complex and the metal-free dye.
The 1:1 chromium complexes containing sulfonic acid groups are made in aqueous solution at 100 °C or at up to 140 °C under pressure for several hours by using an excess of a chromium salt of a strong acid such as HCl, H2SO4, or HF. In the case of azo compounds devoid of sulfonic acid groups it is valuable to add or to employ exclusively an organic solvent, such as alcohol, ethylene glycol, monoalkyl ethylene glycol, or monoalkyl diethylene glycol. The use of a solvent allows higher reaction temperature and shortened reaction time without the need to use pressure equipment. The resulting 1:1 chromium complexes having one sulfonic acid group can be conveniently isolated as insoluble betaines 2, which become slightly water soluble above pH 4 in the form of salts of sulfonic acids 3 (Scheme 2.10).
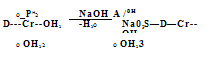
![]() OH
OH
HO3S—D + CrCl3-6H20 OH
Scheme 2.10 Chromium 1:1 complexes as unsoluble betaines 2 and as slightly soluble sodium salts of sulfonic acids 3.
Decreasing acidity of the reaction medium favors the formation of 1:2 chromium complexes, for example, by using strongly buffering form amide as solvent or by using chromium salts of buffering weak acids, in general acetic acid. Under conditions yielding 1:2 chromium complexes no 1:1 complex can be detected during the metallization, and this indicates that the second complex-formation step proceeds more rapidly than the first. This fact can be utilized in the synthesis of 1:1 chromium complexes by first making the 1:2 complex above pH 4 and then treating it with mineral acids to obtain the 1:1 complex [8].
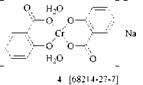
Metallizable azo dyes having no water-solubilizing groups such as sulfonic acid, carboxylic acid, alkylsulfonyl, or sulfonamide are sparingly soluble in aqueous acidic medium and can not be chromed completely under these conditions. One method to overcome this difficulty is the addition of alcohols [9] and carbo — namides [10]. Another method is the treatment of the azo dyes in alkaline solution to yield conventional 1:2 complexes. In this case a chromium complex of a chelating organic acid, such as oxalic acid [11], tartaric acid [12], or salicylic acid [13], or a mixture of chromium salt and chelating acid serves as chromium source.
Using these agents, especially the particularly effective 1:2 complex 4 with salicylic acid, metallization can be carried out easily at high pH without precipitation of inert chromium hydroxide. In certain cases a 1:1 chromium complex that still contains chelating organic acid as ligand can also be obtained.
|
|
An alternative method of preparing 1:2 chromium complexes under alkaline aqueous conditions is based on the use of an alkaline dichromate-glucose solution. In this mixture at boiling temperature, first CrVI is reduced to Cr11 with a reactive d4 configuration followed by rapid formation of the 1:2 Cr11 complex and then the 1:2 Cr111 complex with partial reductive fission of the azo chromophore. The partial dye decomposition is apparent in the decrease of tinctorial yield compared to chromation with Cr111 reagents. Introduction of chromium by simultaneous demethylation requires the use of sodium dichromate and the high-boiling solvent glycol, which at 120-150°C also serves as a reductant for CrVI.
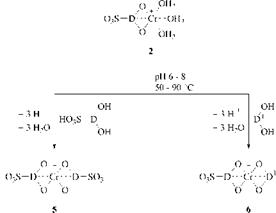
The formation of 1:2 chromium complexes can also be performed in two steps, first by production of the 1:1 complex and then by treating it with a equimolecu — lar quantity of a further metallizable dye to give a 1:2 complex [14]. This complex is symmetrical, for example, 5, if the added dye is identical with the 1:1 chromium-based dye, and unsymmetrical, for example, 6, if this is not the case (Scheme 2.11).
|
Scheme 2.11 Stepwise synthesis of symmetrical 5 and unsymmetrical 1:2 chromium complexes 6 |
The latter stepwise method is the method of choice for the production of the commercially important 1:2 chromium complexes having only one sulfonic acid group (6). This class of metal-complex dyes is made by converting the sulfonic acid bearing dye to the 1:1 chromium complex, isolating the resulting betaine and addition of the sulfonic acid-free counterpart in aqueous weak alkaline solution under mild conditions at 50-90 °C.
Unsymmetrical 1:2 chromium complexes are also obtained in one step by so — called mixed metallization. This means 1:2 chromation of a mixture of at least two different metallizable dyes, leading in the case of two dyes to a mixture of two symmetrical 1:2 complexes and an unsymmetrical 1:2 complex as main products. The mixture is statistical if the affinity of the two metallizable dyes for chromium is comparable; otherwise, the two symmetrical 1:2 complexes prevail. The mixed method is technically exploited for producing the important class of 1:2 chromium complexes having a solubilizing group, such as sulfonamide or alkyl — sulfonyl, on each dye. Moreover, the method is very versatile. For extending the range of shades and matching a definite shade, one can increase the number of metallizable dyes or change their molecular proportions to another. For example, a mixture of 4 mol of dye A, 3 mol of dye B, and 3 mol of dye C with 5 mol of a chromation agent furnishes in statistical distribution the symmetrical and unsymmetrical 1:2 chromium complexes as outlined in Scheme 4, and 1:2-chromation of a mixture of 10 metallizable dyes yields 10 symmetrical and 45 unsymmetrical 1:2 chromium complexes.
5A + 3B + 2C + 5Cr
4
1. 25 ACrA + 0.45 BCrB + 0.2 CCrC + 1.5 ACrB + 1 ACrC + 0.6 BCrC
Scheme 2.12 Products of the mixed 1:2-chromation of three dyes in the molecular proportions 5:3:2
Cobalt Complexes. Preparation of 1:2 cobalt complexes does not require such high reaction temperatures as the corresponding 1:2 chromium complexes, since the aqua cobalt complexes are less inert than those of chromium. The usual method is the reaction of Co11 salts in alkaline medium at about 60 °C, which leads rapidly to the diamagnetic 1:2 Co111 complexes. Atmospheric oxygen serves as oxidant. If the reaction is carried out under anaerobic conditions or if the cobaltization proceeds too slowly, partial reductive fission of the azo dye results. The usual remedy to minimize dye degradation is dropwise addition of dilute hydrogen peroxide simultaneously with the cobalt(ii) salt solution. Direct use of Co111 is unfavorable, because it acts as strong oxidant and forms the 1:2 cobalt(iii) complex more slowly than Co11. Demethylative cobaltization is achieved in high — boiling glycols above 100°C in the presence of a base such as alkanolamines or sodium hydroxide.
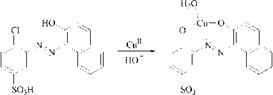
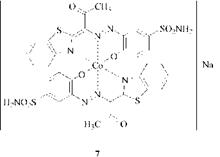
Co111 exhibits a greater tendency to form complexes with nitrogen-donor ligands than with oxygen-donor ligands. Thus the azo dye from diazotized 2-amino- 1-hydroxybenzene-5-sulfonamide and acetonylbenzthiazole affords the 1:2 cobalt complex 7, which is coordinated with the ring nitrogen of the thiazole nucleus and not with the enol oxygen atom of the acetyl group as described in [15]. An indication for the involvement of the ring nitrogen atoms is the brown shade of 7.
|
|
In contrast to chromium, triaqua 1:1 cobalt complexes cannot prepared in acidic medium. Owing to the high affinity of nitrogen for the Co™ ion, 1:1 cobalt complexes of tridentate dyes can be obtained when the coordination requirement is satisfied by nitrogen ligands [16]. The reaction is achieved, for example, in presence of a large excess of ammonia to give triammino 1:1 cobalt complexes. These complexes are insufficiently stable for technical application, but they can readily be converted with an equimolecular quantity of another metallizable azo dye to an unsymmetrical 1:2 cobalt complex [17]. Nitrite, as a strong nucleophile, also stabilizes intermediates for the production of unsymmetrical 1:2 cobalt complexes [18]. Bidentate nitrogen donor ligands, such as ethylenediamine, biguanide, and dipyridyl, are even more suitable for stabilizing 1:1-cobalt complexes [19], and tridentate nitrogen-donor ligands, such as diethylenetriamine and terpyridyl, are even better [20]. Complexes of this type are too stable to be converted into 1:2 cobalt complex dyes.
Iron Complexes. Iron complexes of tridentate o, o’-dihydroxyazo compounds are prepared under weakly acidic conditions at 40-80°C. Both Fe11 and Fem salts can serve as iron source. The Fe111 complexes that result in both cases do not have sufficient stability to dye textile substrates, but the dyeings on leather have good fastness properties [21].
1-Nitroso-2-hydroxyaryls always form 1.3 Fe11 complexes with Fe11 and Fe111 salts. The 1:3 stoichiometry, that is, three bidentate nitrosohydroxy ligands around one Fe11 ion, is readily revealed by metallization of a mixture of two different compounds, for example 1-nitro-2-naphthol-6-sulfonic acid (A) and 1- nitroso-2-hydroxy-6-sulfoamide (B). In this case four products can be discriminated on the chromatogram in the following order: type AAA, type AAB, type ABB, and type BBB.
 4 сентября, 2015
4 сентября, 2015  Pokraskin
Pokraskin 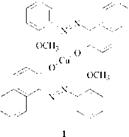
 Опубликовано в рубрике
Опубликовано в рубрике 