![]()
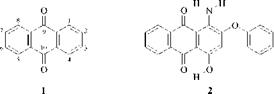 |
(1, 4, 5, and 8). The most common substitution patterns are 1,4, 1,2,4, and 1,4,5,8-. To optimize the properties, primary and secondary amino groups (not tertiary) and hydroxyl groups are employed. These ensure the maximum degree of л-orbital overlap, enhanced by intramolecular hydrogen-bonding, with minimum steric hindrance. These features are illustrated in C. I. Disperse Red 60 (2).
The strength of electron-donor groups increases in the order: OH<NH 2<NHR<HNAr. Tetrasubstituted anthraquinones (1,4,5,8-) are more bathochromic than di- (1,4-) or trisubstituted (1,2,4-) anthraquinones. Thus, by an appropriate selection of donor groups and substitution patterns, a wide variety of colors can be achieved.
 22 августа, 2015
22 августа, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 