Rutile titanates represent by far the largest commercial class of CICPs. Chromium antimony titanate yellows (C. I. Pigment Brown 24) are the most widely used, followed by nickel antimony titanate yellows (C. I. Pigment Yellow 53). Manganese antimony titanate browns (C. I. Pigment Yellow 164) occupy a much smaller market share, and the other rutile grades a significantly smaller fraction still.
Rutile CICPs contain a significant amount of titania as a base oxide. Typically they range from 70 to 90% TiO2 by pigment weight. Transition metal cations Ni(II), Cr(III), and Mn(III) are responsible for producing the color, while the colorless ions Ti(IV), Sb(V), Nb(V), and W(VI) are present to maintain the MO2 stoichiometry. Colors range from light yellow to dark brown. Reflectance curves for the three antimony titanates are compared to rutile TiO2 in Figure 5.4.
It is well known that pure rutile titanium dioxide has a photoactive surface [5, 6]. When irradiated with UV light, highly reactive oxo radicals are formed that can photocatalytically degrade organic materials in contact with the pigment’s surface. Commercial grades of rutile TiO2 are passivated with coatings of other metal oxides such as those of aluminum, silicon, or zirconium to suppress this effect.
Rutile CICPs do not exhibit photocatalytic activity as do pure titanium dioxides. The act of doping the rutile structure with the transition metal and other ions
|
Wavelength in nanometers Figure 5.4 Reflectance spectra of selected rutile CICPs compared to rutile TiO2. |
eliminates the mechanism for formation of surface radicals via UV irradiation. Commercial grade rutile CICPs do not require surface coatings to make them inert.
5.4.2
 3 сентября, 2015
3 сентября, 2015  Pokraskin
Pokraskin 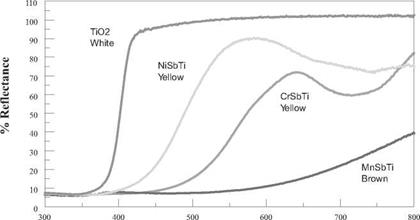
 Опубликовано в рубрике
Опубликовано в рубрике 