3.3.1
Cadmium
Cadmium is positioned in Group Ilb of the periodic table, which is sometimes known as the “zinc group”. It is positioned between zinc and mercury, and thus has some similar properties to these elements.
Cadmium metal is silvery white and reasonably soft. Although it does form some complexes, its pigment chemistry is entirely concerned with the +2 state.
An important property is that, because of its volatility (melting point 321 °C, boiling point 765 °C), cadmium metal can be burned in air to form a brown oxide (CdO). This is not stable in moist air and will slowly convert to the carbonate. However, CdO is readily soluble in mineral acids and is used as a starting point to make solutions of cadmium salts for pigment manufacture.
The sulfate, nitrate and chloride salts are all water soluble, but the white carbonate, hydroxide and phosphate salts of cadmium are insoluble in water.
Yellow cadmium sulfide is highly insoluble (more so than zinc sulfide) and can be precipitated from acidic solutions. Cadmium selenide is almost black and has no pigmentary properties, but is used in the semiconductor industry.
The solubility products of CdS and ZnS are 8 x 10-28 and 1.6 x 10-24, respectively. It is this property that sets cadmium pigments apart from other cadmium salts.
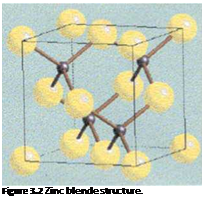
Like zinc sulfide, cadmium sulfide has two forms, cubic (zinc blende, see Figure 3.2) or hexagonal (wurtzite, see Figure 3.3).
 |
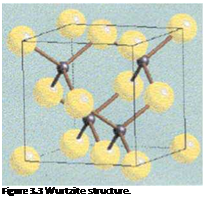 |
The cubic form is usually precipitated, but is not thermally stable and can be converted to the brighter, more stable hexagonal form on heating.
 26 августа, 2015
26 августа, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 