The majority of processes aimed at obtaining rare earth sesquisulfides of high purity use solid/gas reactions.
Most of the methods of synthesizing lanthanide sulfides involve high-temperature gas-solid reactions between a sulfurizing agent (H2S and/or CS2) and lanthanide precursors at high temperatures or under high pressures [21, 22].
Various types of lanthanide precursors can be used: oxides, salts, alkoxides, or organic complexes like oxalates, carbonates, tartrates and malonates [23-27].
Solid-state reaction involving direct treatment of the precursor with elemental sulfur is also possible. In this case lanthanide metal can be used. Processes of this type have the disadvantage of being either difficult or impossible to be commercialized because:
1. They require excessive temperatures and/or pressure conditions.
2. They involve precursors incompatible with the production of sesquisulfides with good coloristic properties and particle size.
Rhodia has now developed and commercialized a special process to produce rare earth sesquisulfides, notably cerium sesquisulfides with the adequate purity and size necessary for pigment application.
Cerium sulfide pigments are prepared in three steps (Figure 4.7):
1. Synthesis of a cerium based precursor
An acidic solution of cerium salt is transformed by a liquid process into a reactive cerium precursor as cited above. At this step, alkali metal and/or alkaline earth metal salts can be added depending on the color targeted. The resulting precipitate is not yet colored, and must next be transformed into a colored sulfide.
2. Sulfuration of the cerium precursor
The transformation of the cerium precursor into the colored sesquisulfide is carried out at high temperatures (between 700 and 1100 °C) in a sulfurizing atmosphere. The resulting colored sesquisulfide is then cooled to room temperature.
3. Final treatment
Once the colored powder is obtained it is ground in order to break all the agglomerates that may have been formed during the sulfurization process. This step is necessary to reveal the pigmentary nature of the pigment. A surface coating is then applied to the Ce2S3 particles by a wet process in order to increase the stability of the pigment and to optimize its compatibility with the final matrix. The coating can be mineral and/or organic (Figure 4.8). Finally, the pigment is filtered, dried, and sieved before packaging.
|
|
|
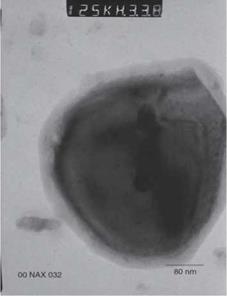
Typical products have the following characteristics:
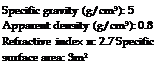 Oil absorption (g/100g): 20-28 Hardness (Mohs): 4-5 Typical particle sizes (pm): 0.8-1.3 Thermal stability: 350-400 °C
Oil absorption (g/100g): 20-28 Hardness (Mohs): 4-5 Typical particle sizes (pm): 0.8-1.3 Thermal stability: 350-400 °C
|
Because of the affinity of metallic rare earths for sulfur, strong bonds are formed in the manufacturing process, inducing high refractory character and chemical inertness in many materials. In air, cerium sulfide pigments are stable up to 350-400 °C, which makes them good candidates for high-temperature processing applications.
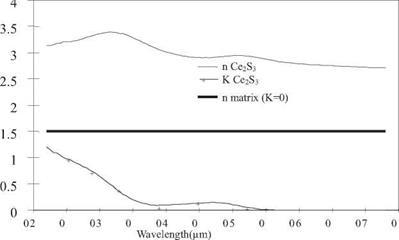
Because of the orbital transition, Ce2S3 materials, like other cerium compounds, are naturally good UV absorbers (Figure 4.9). No photocatalytic effect is observed.
 1 сентября, 2015
1 сентября, 2015  Pokraskin
Pokraskin 
 Опубликовано в рубрике
Опубликовано в рубрике 