A comprehensive patented range of titanates is marketed by Kenrich Petrochemicals, Inc. under the trade name Ken-React, and most of the published data on titanates emanates from this source. It is claimed that a typical titanate coupling agent provides six functions [17], although only three may be considered relevant to their use as adhesion promoters: the reaction of the alkoxy group of the titanate with free protons on the mineral surface to form an organic monomolecular layer on the substrate; transesterification resulting in cross-linking with carboxyl and hydroxyl groups in the polymer; and possibly chain entanglement. Titanate coupling agents are unique in that their reaction with free protons on the substrate surface results in a monomolecular layer
on the mineral surface whether it be filler particle or metallic substrate. The reaction proceeds according to the equation
R0O = Ti(OR)3 + M = OH! MOTi(OR)3 + ROH (6)
where RO is a hydrolyzable moiety. Cassidy and Yager [48] speculate that ester linkages are hydrolyzed and coordination or condensation occurs between the resulting hydroxyl groups and substrate surface groups. Calvert and co-workers [49] infer the presence of strong bonds between isopropoxytitanium tristearate and SiO2 and Al2O3 by the failure to remove the coupling agent by an extended hot-water treatment.
X-ray photoelectron spectroscopy studies by Yang and co-workers [50] showed that aluminum and steel surfaces treated with di(dioctyl)pyrophosphate were covered with the titanate coupling agent, and in the case of steel, the octyl groups of the titanate molecule were uppermost, confirming the view that titanates modify hydrophilic metal oxide surfaces with a hydrophobic organic layer. The possibility has been raised that acidic surface sites on glass may catalyze condensation with surface sila — nols when chelate titanium acetyl acetonate is used [8]. The range of chemical types include monoalkoxy, chelate, coordinate, neoalkoxy, and cycloheteroatom. A very few of the wide range of commercial titanate coupling agents available are shown in Table 4.
Although there are many references to the improvement in adhesion of surface coatings obtained by the use of titanates, numerical data are sparse [51] and few are available on their use in conjunction with adhesives. The literature contains conflicting evidence on the value of titanate adhesion promoters, and in an investigation of eight titanates tested with an acrylic resin only two titanates performed better than the nontitanate control, It has been claimed that alkyl titanates are effective coupling agents for polyethylene [52-54]. Using isopropyl triisostearoyl titanate as a primer
Table 4 Typical Titanate Coupling Agents
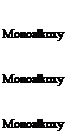
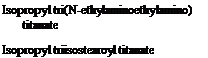 |
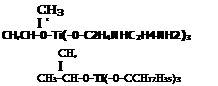 |
 |
|
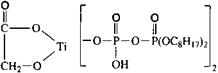
Chemical Description Structure Type
![]()
![]()
![]() Tetraisopropyl di(dictylphosphito) titanate
Tetraisopropyl di(dictylphosphito) titanate
Neoalkoxytri[ p-N-(b-aminoethyl)amino phenyl] titanate
for polyethylene/Al2O3 joints, Sung and co-workers suggested that it was unlikely that this particular titanate functioned as an adhesion promoter in this system, notwithstanding the observation that heating the titanate above 70°C in vacuo resulted in a significant increase in peel strength [55].
Calvert and co-workers [49] have demonstrated the presence of isopropyl isostearate and isopropyl laurate in commercial isopropyl triisostearoyl titanate and conclude that this is the reason why the commercial product does not function as an adhesion promoter; treatment at 70°C in vacuo removes these fatty acid esters. It has been suggested to the author that failures to obtain improvements in adhesion using titanates can be remedied by isolating the pure compound (B. Nordenheim, private communication, 1988). It is possible that improvements in adhesion are more likely when commercial titanates are used as additives rather than as pretreatment primers.
It is only fair to say that the trade literature [17] is emphatic that it is critical to use the correct amount of titanate coupling agent. The use of excessive amounts is probably the most significant factor in application failure tests. It is strongly recommended that selected titanates should be examined in a range of concentrations from 0.1 to 2.0% by mass in a filled system and even lower for unfilled systems. Excess titanate will result in unreacted alkoxy groups on the surface and in a loss of adhesion of the polymer. This could lead to the mistaken conclusion that a particular titanate was unsuitable or even harmful.
In general, titanates with the more polar organic moieties such as isopropyl tri(N-ethylenediamio)ethyl titanate and neoalkoxytri[-P-N-(p-aminoethyl)amino phenyl] titanate are recommended for adhesion promotion to polar substrates. Titanates with relatively nonpolar moieties, such as aliphatic carboxy titanates and isopropyl tri(dioctylphosphato)titanate, will adhere better to the nonpolar substrates.
 3 июля, 2015
3 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 