A. The Thermodynamic or Reversible Work of Adhesion
In the absence of chemisorption and interdiffusion, the work of adhesion is the sum of the various intermolecular forces involved and can be related to the surface free energies (Dupre’s equation):
W = Yi + Y2 — y12 (8)
where у 1 and y2 are the surface free energies of components 1 and 2, and y12 is the interfacial free energy. For two materials interacting via London dispersive forces only across their interface, Fowkes [4] suggested that W be described by
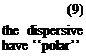 W=W d = 2(y dyd)1/2
W=W d = 2(y dyd)1/2
where Wd is the dispersive contribution to the work of adhesion and yd contribution to the surface energy y,. In the case where both materials interacting sites, W can be described by
W = Wd + Wp (10)
where Wp is the polar contribution to the reversible work of adhesion. Wp was described by [53]
Wp = 2(yp yf)1/2 (11)
where y, p is the polar contribution to the surface energy of the, th species. This is known as ‘‘the extended Fowkes equation.’’ However, Fowkes [13,54] has demonstrated that Eq. (11) is incorrect and cannot predict the magnitude of the nondispersive interactions. The main problem of the ‘‘extended Fowkes equation’’ is the wrong assumption that the nondispersive contribution to W of two polar materials can be represented by the geometric mean value of their polar properties. Indeed, when Eqs. (8)-(11) are applied to a liquid-liquid system, such as water-ethanol, it cannot predict their miscibility or immis — cibility. Although the yp value for ethanol is only 1.1 mJ/m2, this liquid is very hydrophilic and miscible in water in all proportions. Fowkes has shown that the use of the geometric
mean expression for estimating the work of adhesion and interfacial tension between water and ethanol predicts these two liquids to be completely immiscible with an interfacial tension of 37.7 mJ/m2, which, of course, is contrary to the physical reality. For this reason, yp is usually a very inadequate measure of polarity or hydrophilicity [13]. It was instead suggested that the nondispersive contribution to the work of adhesion attributed to Lewis acid-base interactions (WAB) could be evaluated by
WAB = W — 2(ydyd)1/2 (12)
Two methods were developed to determine WAB: the first was suggested by Fowkes and Mostafa in 1978 [11] and the second approach was introduced by van Oss and co-workers in 1988 [22].
 23 июня, 2015
23 июня, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 