For moderately rough surfaces, an increase in surface area may well lead to a proportionate increase in adhesion, so long as the roughness does not reduce contact between the surfaces. Gent and Lai have convincingly demonstrated the effect in careful experiments with rubber adhesion [65]. In comparing adhesion to smooth and to grit blasted steel, they observed increases in peel energy by factors of two to three times which they ascribed to the increase in surface area. This is consistent with the concept of the Wenzel roughness factor, and many authors would discount this as coming within the scope of the mechanical theory of adhesion.
A classic instance of the mechanical theory of adhesion is where one phase is ‘‘keyed’’ into the other. Here the adhesion is enhanced above the increase proportional
to the surface area by exploiting the mechanical properties of the ‘‘keyed’’ material (strength or toughness) in enhancing the measured adhesion. There are many descriptions of this in the literature. A simple example is provided by the adhesion of silica to copper discussed by van der Putten [66], who was concerned to bond copper directly to silicon in the context of integrated circuit technology.
Copper sticks poorly to silica but titanium tungstide sticks well. Using conventional lithographic techniques islands of TiW 0.1 pm thick, a few micrometers in width, were sputtered onto the silica and the photoresist was removed (Fig. 5(a)).
Palladium acts as a nucleating agent for the electroless deposition of copper. By treating the surface with palladium [II] chloride in hydrochloric acid a monolayer or so of palladium is deposited on the TiW surface. The palladium chloride solution also contains 1% of hydrofluoric acid which attacks the silica, undercutting the TiW islands (Fig. 5(b)). Electroless copper is now deposited, nucleating on the palladium-covered TiW and growing from it. Finally copper is electrodeposited and is thus mechanically anchored to the silicon surface (Figs. 5(c) and (d)).
Here the stress is directed away from the low Wa interface (silica/copper) towards the stronger silica/palladium interface by the topography produced. The surface topography protects weak regions from a high stress field.
Another example may be cited from the field of polymer-polymer adhesion. When sheets of semicrystalline polymers, such as polypropylene and polyethylene, are laminated by cooling from the melt, a key may form. There are examples where the lower-melting polymer has been shown to flow into the structure of the higher-melting material as its volume contracts on crystallization [67-70]. These influxes, which may be hundreds of
|
Figure 5 Adhesion of copper to silica using a mechanical key: (a)-(d) successive stages (see text) (after Ref. 66). |
micrometers in size, lead to a mechanically reinforced interface associated with enhanced adhesion (cf. Eq. (6)).
 21 июня, 2015
21 июня, 2015  Malyar
Malyar 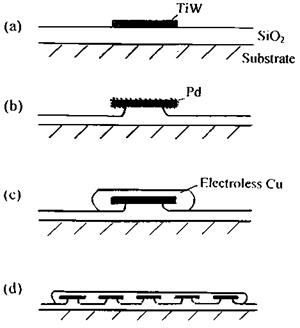
 Опубликовано в рубрике
Опубликовано в рубрике 