The reactions of interest in silane coupling are summarized below.
(a) Hydrolysis of the silane group: pH or catalyst
R“SiX, + 3H20—————————————— ► R-Si(OHb +3HX ( 1
where HX is usually an alcohol.
|
Table 2 Typical Silane Adhesion Promoters Commercially Available
|
|
|
|
|
|
|
 |
|
|
|
![]()
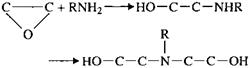 (5)
(5)
In this case reaction of the primary amine group in an aminosilane with an epoxide group.
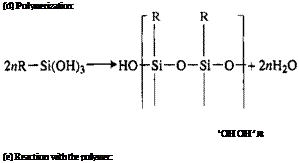
Hydrolysis, Eq. (1), may take place on the surface by reaction with surface water or in solution prior to application, occurring rapidly in neutral or slightly acidic water solutions and only slowly in hydrocarbon solvents [31]. Aminosilanes are autocatalytic and do not depend entirely on hydrolysis for aqueous solubility [32]. Polymerization, Eq. (4), may occur not only on the surface as the silane triols, RSi(OH)3, condense to form oligomeric siloxanes as in Eq. (3) via disiloxanols and trisiloxanols but also in solution before application. The speed at which this occurs and the oligomers become insoluble depends on silane concentration, solution pH, the presence of soluble catalytic salts [16], and the type of silane [33]. The pH factor is particularly important in silane technology.
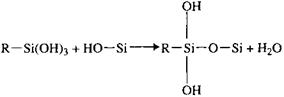
The hydrolyzed silanol group will react with inorganic surface hydroxyl groups to form hydrogen bonds, Eq. (2), followed by condensation to form oxane bonds, Eq. (3). It should be noted that both hydrogen and oxane bond formation is reversible. Equation (5), reaction with the polymer, is a typical example of many possible reactions, depending on the functional groups on the silane and the polymer. In work with surface coatings,
Walker [34] has postulated a variety of possible chemical reactions between silanes and the functional groups present in epoxide and polyurethane compositions.
 3 июля, 2015
3 июля, 2015  Malyar
Malyar 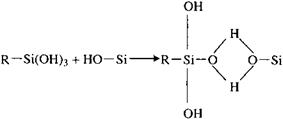
 Опубликовано в рубрике
Опубликовано в рубрике 