The molecular probe technique in combination with XPS has seldom been used since the early 1970s and has mainly been applied to zeolites [162-164]. These studies were aimed at identifying and quantifying Lewis acidic and basic sites at catalyst surfaces by monitoring the BE shifts of N1s from adsorbed pyridine [162,163] and pyrrole [164], respectively. We shall discuss the application of this approach to molecular and polymeric species.
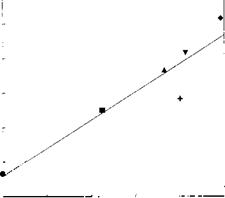
a. Molecules: Relationship with Gutmann’s and Drago’s Acid-Base Constants. Burger and Fluck [165] established, for quickly frozen solutions of SbCl5-Lewis base complexes in 1,2-dichloroethane, a linear relationship between Sb3d5/2 BE and DN, the donor number of the complexing Lewis bases. On this basis, Chehimi [166] showed that the Sb3d5/2 BE was linearly correlated with the ДИАВ of (base-SbCl5) adduct formation calculated using Drago’s equation. Figure 16 depicts a linear correlation of Sb3d5/2 BE versus ДИАВ (base-SbCl5). Therefore, XPS is a potential tool for estimating Drago’s parameters for polymer surfaces [166], which have actually been confirmed for PPO [167] and plasma-treated polypropylene [46] (see Table 3).
b. Polymers: Sorption of Specific Probes. Chehimi and co-workers
[154,166,168-171] established protocols to quantitatively estimate the acid-base properties of polymer surfaces using (ad)sorbed molecular probes. In practice, a polymer is exposed to liquid vapors (solutes) of known acid-base properties for a few minutes. The polymer is then allowed to outgas the excess of solute and is transferred into the XPS equipment for surface characterization. If the polymer-solute interaction is strong enough (e. g., via acid-base forces) then a residual amount of solute is detected and quantified.
(i) Choice of Molecular Probes. Several molecular probes can be used to characterize the acid-base properties of solid surfaces by XPS. For example, chlorinated and
|
|
|
|
|
|
|
|
|
|
Table 12 Molecular Probes Used for XPS Determination of Acid-Base Properties of Materials
|
fluorinated acidic species probe Lewis basicity, whereas pyridine [168,154,163] or DMSO [83] are suitable to characterize surface acidity (Table 12).
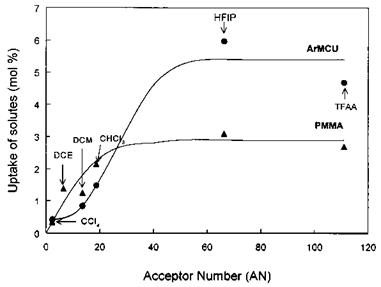
Figure 17 depicts a survey scan of a basic aromatic moisture-cured urethane resin (ArMCU) before and after exposure to the vapors of hexafluoroisopropanol (HFIP) (CF3CH(OH)CF3), a reference Lewis acid. The F1s from HFIP is easily detected indicating that it was retained by the ArMCU. This retention, despite the high vacuum, is believed to be governed by acid-base interactions between the OH group from HFIP and the carbamate (HN-C = O) group from the resin. The molar ratio of solute per polymer repeat unit (%S, where S stands for solute) was evaluated and used as a measure of the uptake (or retention) of solute by the host polymer. Figure 18 shows plots of
|
|
|
%S versus AN (Gutmann’s acceptor number) of the solute, for the host polymers PMMA and ArMCU. The plots are S shaped, showing an increasing uptake of solute with AN, which denotes the basic character of both polymers. This is in agreement with Fourier Transform Infrared (FTIR) studies of the Lewis basicity of PMMA [13] and ArMCU [170]. In contrast, XPS did not detect any retained chloroform at the polyethylene surface following exposure to the vapors because the polymer-solute interactions reduce to London dispersive forces only.
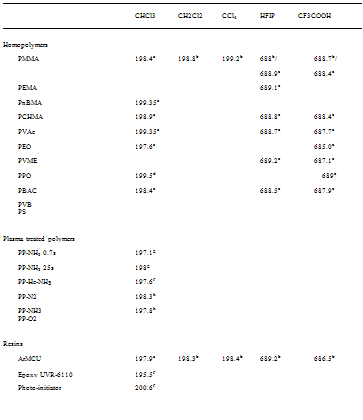
(ii) Chemical Shifts of the Molecular Probes. The binding energies (BEs) of core electrons from the solutes’ elemental markers were also investigated for various polymers and resins (Table 13). Chloroform has been the most extensively used Lewis acid to characterize polymer basicity. Table 13 shows that acidic (CHCl3 and CF3Ca) and basic (pyridine) probes undergo negative and positive chemical shifts (lower and higher BEs), respectively, when they interact with host surfaces via acid-base forces. Indeed, a Lewis acid is an electron acceptor and upon interaction with a base via acid-base forces, electron density is transfered to the Lewis acid thus yielding a lower binding energy of its electrophilic site [43,168,169]. The opposite reasoning holds for basic probes [154,168].
Table 13 Binding Energy (eV) for Molecular Probes Adsorbed onto Polymers and Resins
![]()
![]()
![]() I2 Pyridine DMSO
I2 Pyridine DMSO
399b 399.7e
168.81
,h h
166.5-168[4]
|
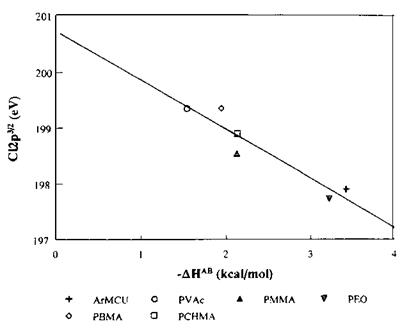
Figure 19 Cl2p3/2 BE versus ДНАБ for CHCl3 sorbed in ArMCU, poly(vinyl acetate) (PVAc), poly(methyl methacrylate) (PMMA), poly(ethylene oxide) (PEO), poly(butyl methacrylate) (PBMA), and poly(cyclohexyl methacrylate) (PCHMA). |
in the high vacuum, there was a continuous desorption of the probe (the purple color was vanishing) leading to a very weak I3d5/2 peak intensity. Nevertheless, the I3d5/2 peak recorded during desorption shifted from 621 to 619 eV, thus towards a lower binding energy. This indicates that the strongly adsorbed iodine molecules were subject to electron density transfer from PS thus leading to low BE. Perhaps PS was not a strong enough Lewis base to retain adsorbed iodine in the high vacuum. Nevertheless, the ESCA group led by J. J. Pireaux in Namur was more successful in characterizing the basicity of plasma — treated polypropylene by iodine vapor [46,172].
(vi) Pyridine. Pyridine is a molecular probe for the acidic sites of catalysts [173]. When adsorbed on polymer surfaces, the N1s core electron undergoes a +1 eV chemical shift (in comparison to the N1s BE for pure pyridine) in the case of the host PMMA owing to the donation of electron density from pyridine to the carbonyl carbon of the methacrylate repeat unit (acidic site) [168]. The N1s chemical shift is even larger (+1.7 eV) when pyridine is sorbed in PVB since it is predominantly acidic due to its OH pendent groups. The N1s BE positions for pyridine-polymer complexes are higher than those of the pure pyridine because the pyridine-pyridine interaction occurs via dispersive forces only, for pyridine is a monofunctional species [7,54].
 25 июня, 2015
25 июня, 2015  Malyar
Malyar 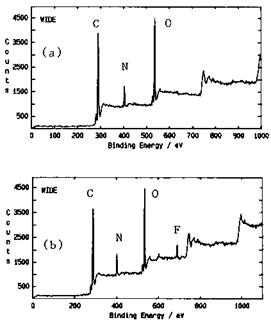
 Опубликовано в рубрике
Опубликовано в рубрике 