From the earliest days, the materials that we later called cements, glues, gums, mucilage, mortars, resins, pastes, and finally, adhesives and sealants, were used interchangeably. Only in modern times have we attempted to differentiate between adhesives and sealants. For the most part it has been a vain attempt, as many so-called adhesives also serve as sealants, and all sealants have adhesive properties. Some polyurethane and silicone sealants have strength properties similar to those of structural adhesives. Only seals, which have no adhesive properties (gaskets, O-rings, stuffing boxes, etc.), have been excluded from the technical definitions, but even here, seals and sealants are often combined in the literature and in use, as they often perform in similar applications. Mixtures of glycerin and litharge, alone and with additives, were used for many years [5, p. 358] as both an adhesive and a sealant, and are still used in the repair and restoration of older aquariums.
In his book The Technology of Adhesives [6], John Delmonte tells us that the first commercial glue plant was founded in Holland in 1690, that casein glues appear to have been manufactured in Germany and Switzerland in the early nineteenth century, and that the first U. S. patent (number 183,024) on a casein glue was issued in 1876. He mentions that starch adhesives were used on postage stamps when they were first issued in 1840, and that the first U. S. patent (number 61,991) on a dextrin adhesive was issued in 1867.
Before the advent of synthetic resin adhesives, semisynthetic cellulosic materials were developed, but when they were first dissolved in solvents and used as an adhesive is not clear from the literature. “Historically, the first thermoplastic synthetic adhesive (only partly synthetic) was the cellulose ester cellulose nitrate, often called nitrocellulose, and it is still one of the most important. Later, other esters such as the acetate were developed, as well as certain mixed esters ’’ [1, p. 295].
Inorganic sodium silicate adhesives had minor commercial use in 1867, but it was not until 1900 that their use as a glue became of commercial importance as a replacement for starch in the production of corrugated and solid fiber paperboard [5, p. 279]. Very fine silicate frit mixed with phosphoric acid was used as a dental cement [5, p. 376] before the twentieth century. Magnesium chloride inorganic cements were used at least as far back as 1876 in hospital kitchen floors, as they provide resistance to greases and oils [5, pp. 355-356].
There is little agreement in the literature about the dates when various adhesives and sealants were first developed or used in a specific application. This is due to simultaneous developments in many parts of the world and the fact that references in the literature are almost exclusively from the more developed countries. Table 1 show Delmonte’s [6, p. 4] viewpoint on the times of adhesive developments, up to the year of publication of his work. In the accompanying text he notes that ‘‘The developments are tabulated according to their first public disclosure, whether by patent or citation in technical literature.’’
Some experts trace the roots of the first modern adhesives technology to 1839, when Charles Goodyear discovered that a mixture of rubber and sulfur changed from a plastic to an elastic state when heated. In 1843 this process was termed vulcanization by Thomas Hancock, who is believed to have used his hard rubber (Ebonite) for bonding to metals, possibly discovering its effectiveness when trying to remove the mixture from metal containers used in its preparation. As it also bonded to natural rubber during vulcanization, it was used for many years as the only practical means of joining metal to rubber—but it had serious limitations as a thermoplastic [7, pp. 1-3].
The rubber cement used in early rubber-to-metal bonding was a simple dispersion of rubber sheeting in benzene and later toluene or other solvent. It was brushed on the metal
Material
![]()
 Glue from animal bones (patent)
Glue from animal bones (patent)
Domestic manufacture of fish glues (isinglass)
First U. S. fish glue patent
Laminating of thin wood veneers attains commercial importance Vegetable adhesives from cassava flour (F. G. Perkins)
Phenolic resin to plywood (Baekeland-Thurlow)
Blood albumin in adhesives for wood (Haskelite Co.)
Casein glues for aircraft construction
Developments in cellulose ester adhesives and alkyd resin adhesives Cyclized rubber in adhesives (Fischer-Goodrich Co.)
Chloroprene adhesives (McDonald-B. B. Chemical Co.)
Soybean adhesives (I. F. Laucks Co.)
Urea-formaldehyde resin adhesives
Specialty pressure-sensitive tapes: rubber base (Drew-Minnesota Mining & Mfg. Co.)
Phenolic resin adhesive films (Resinous Products & Chemical Co.) Poly(vinyl acetate) adhesives (Carbide & Carbon Chemicals Co.) Chlorinated rubber adhesives
Melamine-formaldehyde resin adhesives (American Cyanamid Corp.)
and Redux by de Bruyne (Aero Research Ltd).
Cycleweld metal adhesives (Saunders-Chrysler Co.) Resorcinol-formaldehyde adhesives (Penn. Coal Products Co.) Metal-bond adhesives (Havens, Consolidated Vultee-Aircraft Corp.) Furane resin adhesives (Delmonte, Plastics Inst.) and Pliobond (Goodyear Tire and Rubber Co.)
Source: Ref. 6.
and dried prior to contact with the bulk rubber to be bonded to the metal by vulcanization in a heated press. In 1862, Charles Sanderson, in a British patent (number 3288), specified that metal be brass plated by electrodeposition to obtain a strong bond to rubber [7, p. 3]. In 1911 the process was used in the production of rubber rolls, but was not used as a general commercial process until the 1920-1930 period.
Efforts to bond rubber to metal without the use of metal plating led to what is believed to be the first research efforts in surface preparation prior to adhesive bonding. Strong and durable bonds of rubber to metal were necessary for rubber shock mounts for automobiles in the late 1920s, but they were limited to proprietary formulations used on specific metals. In 1927 solvent-based thermoplastic rubber cements for metal-to-rubber bonding were prepared from rubber ‘‘cyclized’’ by treatment with sulfuric or other strong acids. With these rubber cements strong bonds could be made to either vulcanized or unvulcanized rubber.
Thermosetting solvent-based rubber cements for rubber-to-metal bonding, based on halogenated rubber compounds, first became available between World Wars I and II, but like much of the rubber-to-metal bonding technology, most of the work was proprietary and only glimpses of the technology involved can be found in the patent literature. The first use of natural rubber-based ‘‘tacky’’ adhesives on a backing is credited to Henry Day, who was issued a U. S. patent (number 3,965) in 1845. James Corbin of Minnesota Mining and Manufacturing Co. (now 3M Company), in a 1952 paper, ‘‘Practical Applications of
Pressure-Sensitive Adhesives’’ [8, p. 139], states that 1925 is generally considered to be the birth date of the pressure-sensitive tape industry. He mentions that prior to the time, both cloth-backed surgical tapes and cloth-backed friction tape for use by electricians were in limited use. Both were apparently tried as masking tapes for the new two-toned automobiles, but failed to resist paint penetration and to strip clean. A crepe-paper backing, impregnated with animal glue and glycerin and coated with a pressure-sensitive adhesive (PSA), was developed in 1925.
Synthetic rubber, a dimethylbutadiene, was developed as a substitute for natural rubber in Germany during World War I and saw limited use as an adhesive. In the early 1930s, neoprene rubber (then called Duprene) became available to adhesive manufacturers in the United States, and shortly thereafter in Great Britain. Today, neoprene rubber adhesives are available as both thermoplastic and cross-linking systems in both solvent and emulsion formulations. Neoprene rubber is the major base resin for contact adhesives. A limited amount of neoprene rubber is also used in sealants.
It was not until the commercialization of synthetic plastics resins in the 1930s that an almost unlimited variety of base materials became available for compounding into adhesives and sealants. Most of the thermoplastic resins were soluble in organic solvents and were used as solvent adhesives for molded plastic articles of the same base composition and sometimes for other materials. Poly(vinyl chloride) (PVC), a thermoplastic developed in 1927, is used today in solvent formulations to bond PVC articles such as coated fabrics, films, foams, and pipe. In the early 1930s, phenolics came into importance as adhesive resins. Before that time they were used as coating varnishes [9, p. 239]. ‘‘About 1931 development of the use of a new phenolic resin for plywoods and veneers began’’ [9, p. 239].
Poly(vinyl acetate) was used as a solvent-based adhesive in the 1930s, and later as a hot melt, but was not of commercial importance until its introduction in the 1940s, as an emulsion adhesive used mainly to bond paper and wood. Today, in emulsion form as a white glue, it is the most widely used thermoplastic adhesive worldwide. Vinyl acetate-ethylene (VAE) emulsion adhesives, with over 55% vinyl acetate content, were developed in the early 1950s but did not become of commercial importance in the United States until the mid-1960s.
Acrylic adhesives first appeared about 1937; ‘‘the acrylic resins may be considered as belonging to the vinyl family’’ [1, p. 305]. Today, acrylic adhesives appear in many forms: as both pressure-sensitive and non-pressure sensitive formulations in organic solvent and emulsion forms; as monomer and polymer cements; as anaerobics; as cyanoacrylates; as so-called reactive or ‘‘honeymoon’’ two-part systems; and as radiation curing formulations. “Commercial production of acrylic polymers began in the late 1920s, but it was not until 1958 that the first acrylic sealant was developed’’ [10, p. 226]. ‘‘The solvent — based acrylic sealants were first introduced to the construction industry in about 1960’’ [11, p. 121].
Urea-formaldehyde adhesives were patented in 1920 but were first commercialized around 1937. During World War II, starch was modified with urea resins to make both waterproof adhesives and impregnants for paper, which led in the 1940s to phenolic — impregnated paper for the first durable honeycomb core for lightweight rigid honeycomb panels.
Prior to World War II only in Germany was bonding to synthetic rubber being done. Polyisocyanate adhesives for rubber-to-metal bonding were developed under Otto Bayer in Germany during World War II. During the war there was widespread bonding of synthetic rubbers to metals in other countries, but documentation is almost nonexistent.
It was only with the development of high-strength toughened phenolic thermosetting adhesives during World War II for metal-to-metal bonding that high-strength bonding of vulcanized rubber to metal became practical. Today, both vulcanized and unvulcanized rubber may be bonded to most materials of commercial importance, with a variety of room — or elevated-temperature setting — or curing-type adhesives.
During World War II, synthetic rubber and resin-modified phenolics were used to bond aluminum sheets (available only in yg-in. thickness at that time) into billets from which airplane propellers were carved, thus replacing laminated wood, which often shattered on impact with a bullet. Similar adhesives were used to bond rubber to metal in a variety of vibration-damping applications. ‘‘The most successful widely known product of the new technology was the automotive bonded brake lining first introduced in 1947, and now regarded as a symbol of quality and integrity’’ [12, p. 490].
In a book entitled Adhesives [2] published in 1943, only six of 150 pages are devoted to synthetic adhesives, and many of these are combined with animal glue and other natural adhesives. There are chapters entitled ‘‘Flour Pastes and Starch Adhesives,’’ ‘‘Dextrin Adhesives,’’ ‘‘Casein Adhesives,’’ ‘‘Vegetable Glues,’’ ‘‘Animal Glues,’’ ‘‘Sodium Silicate Adhesives,’’ ‘‘Rubber Dispersions and Solutions as Adhesives,’’ ‘‘Rosin and Its Derivatives,’’ ‘‘Wax Adhesives,’’ ‘‘Putties,’’ and other chapters on adhesives from natural raw materials. In one chapter, ‘‘Miscellaneous Adhesives,’’ there is a single formulation where a synthetic, poly(vinyl alcohol), is combined with starch. There is one chapter, ‘‘Gums and Resins (Natural and Synthetic),’’ with no mention of any synthetic material, and a single small chapter, ‘‘Adhesives Derived from Synthetic Material,’’ where phenol — formaldehyde, urea-formaldehyde, and acrylic resins are mentioned, which suggests that they can be blended with animal glues to produce strong, waterproof adhesives. Also mentioned are poly(vinyl acetate), used alone or combined with ethyl cellulose. There is no mention of the rubber-modified phenolic adhesives developed during World War II, possibly because such formulations were classified as ‘‘secret.’’
One interesting omission in the book Adhesives is the use of poly(vinyl butyral) as the adhesive in safety glass. In 1936, Carbide and Carbon Chemicals Corporation first describes the use of poly(vinyl butyral) for laminating ‘‘high-test’’ safety glass [13, p. 165]. But in this book, poly(vinyl acetate), used as an adhesive for cellulose nitrate or cellulose acetate film, is mentioned as one laminating material for safety glass. This omission was particularly evident to the author of the present article, as poly(vinyl butyral) was a major product of my employer, E. I. DuPont, at their Plastics Division in North Arlington, New Jersey, in 1941. It had two major uses, as a safety glass laminating adhesive and as a box-toe softener for leather shoes.
To see just how far progress in adhesives and sealants extended during World War II, one has only to compare the book Adhesives with a book completed three years later, in December 1946. The Technology of Adhesives [6] had 516 pages, over 4000 index entries, and 1900 references. It covers in great detail the history, chemistry, theoretical background, testing, and technology of adhesives, It ‘‘seems’’ to have been written decades after the other volume. The term ‘‘pressure-sensitive adhesives,’’ not found in the first volume, has 13 index entries, and similarly, ‘‘hot melts’’ has six index entries. Resorcinol-formaldehyde for wood bonding, introduced commercially in 1943, is covered in detail in the second volume, and an entire chapter, ‘‘Cementing of Organic Plastics,’’ covers both thermoplastic and the thermosetting materials, whereas the other volume mentions neither.
Again, this was of particular interest to the author, as in 1941 I helped with the formulation of a number of the solvent cements for acrylics used in the fabrication and repair of transparent acrylic aircraft enclosures. These adhesives, by the way, are still being sold by a number of vendors and are widely used by sign, incubator, and other fabricators of acrylic plastics. ASTM Committee D-20 on Plastics was organized in the United States in 1937-1938, and adhesives were a regular topic of discussion. From this committee came the nucleus of members who organized ASTM Committee D-14 on Adhesives in 1944.
Silicone adhesives were introduced commercially in 1944 [5, p. 213]. ‘‘In 1960 the silicone sealants were introduced to the construction industry’’ [11, p. 86]. Silicones are useful at both high and low temperatures and are available today as solvent-based moisture-curing adhesives, one-part moisture-curing adhesives and sealants, two-part curing adhesives and sealants, and pressure-sensitive adhesives.
According to one author, epoxy-phenolic adhesives for high-temperature applications were developed during World War II at Forest Product Laboratories in Madison, Wisconsin, and nitrile phenolic adhesives shortly after World War II [9, pp. 153, 156]. A patent for epoxy resins was applied for in Germany in 1934, ‘‘and the inventor disclosed that it could be hardened with equivalent amounts of amines, diamines, or polyamines and that it showed strong adhesion’’ [14, p. 8]. Epoxy resins are believed to have been commercialized in the United States first by the former Jones Dabney Company sometime after 1942.
Polyurethanes had their commercial beginning with the work of Otto Bayer in Germany in 1937. ‘‘In addition, American patent literature revealed that in the early 1940s much study was directed toward the use of di-isocyanates as adhesive assistants, particularly in adhering elastomers to metals and fibers’’ [15, p. 4]. ‘‘…The following working definition of polyurethanes may be derived—they are polymers produced by addition reactions between polyisocyanates (difunctional or higher) and hydroxyl-rich compounds (at least two hydroxyl groups per molecule) such as glycols polyesters, polyethers, etc.’’ [15, p. 3]. Today, polyurethane adhesives are available as solvent-based moisture-adhesives, thermoplastic hot melts, thermosetting systems, and emulsions.
During World War II, from 1939 to 1945, under the pressure of wartime shortages and the development of new and improved weapons of war, great progress was made in adhesives and adhesive bonding. However, due to wartime secrecy, much that went on has never been formally published. The homopolymer polyisobutylene was used in pressure — sensitive adhesives (PSAs) in 1939 as a replacement for natural rubber PSAs. Today, butyl rubber, the copolymer, has minor use in adhesives but is widely used in sealants. Polyvinyl acetals [poly(vinyl formal) and poly(vinyl butyral)] were used as flexibilizers for phenolic resins to make tough metal-bonding adhesives.
Styrene-butadiene rubber (SBR) adhesives, used to replace natural rubber adhesives, saw limited use during World War II, but commercialization took place during the 1950s. Today, in terms of monetary value, SBR adhesives are the most important adhesives in the United States. Their use in sealants is minor.
When glass-fiber reinforcements were used in organic resins in the 1940s, they lost much of their strength during prolonged exposure in water. In 1947 silanes were found to be effective primers or ‘‘coupling agents.’’ ‘‘Silane monomers may be used in integral blends of fillers and liquid resins in the preparation of composites. The modified polymer I ‘adhesive’ in this case is termed a matrix resin’’ [16, p. 4]. In a chapter entitled ‘‘The Chemistry of Tackifying Terpene Resins,’’ we learn that terpene resins were first produced for adhesive applications in the early 1950s, first for pressure-sensitive adhesives and were then combined with wax in early synthetic resin hot melts [17, pp. 396-397].
Anaerobic materials were discovered in the 1940s but were not commercialized until the early 1950s as a new form of acrylic adhesives, termed ‘‘anaerobics’’ by their inventor,
Vernon Krieble, then a professor at Trinity College in Hartford, Connecticut. Their first use was as “threadlocking’’ sealants, to lock nuts on threaded fasteners as a replacement for metal lock washers, and to lock threaded fasteners in tapped holes in metal parts. They were the first products termed ‘‘sealants’’ to have a viscosity lower than that of water. Today, such anaerobic adhesives and sealants are used in almost all mechanical equipment that is subject to vibration.
Polysulfide rubber was first produced in 1929, and the liquid polymers were used in sealants and as flexibilizers for epoxy adhesives around 1950. ‘‘In 1952 the polysulfide sealant was introduced to the construction industry’’ [11, p. 74]. ‘‘In the 1950s the first butyl rubber caulks appeared in the construction market’’ [11. p. 108] and ‘‘latex caulks’’ [vinyl acrylic and poly(vinyl acetate)] appeared sometime after 1956.
Polyester resins (alkyds) were commercialized for coatings use in 1926, and unsaturated polyesters were used as thermoset fiberglass composite matrix resins in the 1940s, but the early resins made poor adhesives. When flexibilized resins appeared in the 1950s, they were used as adhesives. Today, unsaturated polyesters are widely used as adhesives for thermoset plastics bonding, and even for metal bonding in most countries, but are seldom used as adhesives in the United States, where the more expensive epoxy adhesives are used in similar applications. The saturated polyesters, used as thermoplastic hot-melt adhesives, seem to have appeared in the literature first in the 1954-1957 period.
Polyethylene seems to have been mentioned first for use in a hot-melt adhesive in a 1954 patent application. Patent 2,894,925 was issued in 1959 [18, p. 62]. Today, polyethylene is the most important of the hot-melt adhesives in terms of tonnage, and is second, after ethylene-vinyl acetate (EVA), in dollar value in the United States. EVA (containing less than 55% vinyl acetate) adhesives, developed in the late 1960s, ‘‘wet’’ more substrates, had better low-temperature properties, and were compatible with more formulating ingre — dients—but all at a higher price.
By the early 1960s, the raw materials used in adhesive formulations were so numerous that in the first handbook on the subject, the editor said: ‘‘It would be a virtual impossibility for any single volume to list all the ingredients which might conceivably be employed in an adhesive compound. Such a list would encompass practically every known chemical compound currently available in the United States’’ [19, p. 11].
It was only in the late 1950s and early 1960s that raw material suppliers established marketing programs that specifically targeted the adhesives industry. Before that time, a person formulating adhesives of more than a single chemical type had to have an extensive knowledge of the product lines of hundreds of supplier firms. For this reason, almost all formulators had backgrounds in the coatings or rubber industries. Many of today’s adhesive manufacturing firms reflect the earlier period by combining adhesives and sealants with the coatings or rubber areas of their businesses. Almost all synthetic resins used in adhesive formulations were used previously in coatings or rubber technology. The few people in the adhesives technical areas that were not from the coatings or rubber areas were mechanical engineers, who could evaluate the physical properties of the compounds developed by the chemists and the strength and durability of bonded assemblies.
From the chemists has come the classification of adhesives and sealants by chemical type, and from the mechanical engineers the classification as either ‘‘structural’’ or ‘‘nonstructural.’’ Neither is a ‘‘pure’’ system, since many adhesives and sealants have more than a single chemical base resin, and many ‘‘structural’’-based resin systems are used in nonstructural applications. In a chapter entitled ‘‘Structural Adhesives’’ we are told that the term structural adhesive came into general use in the 1960-1970 period, but to this day all definitions are inadequate [9, Chap. 7]. ‘‘Adhesive manufacturers and their advertising departments now miss no opportunity to use, or abuse, the word. Companies which formerly sold urethane, acrylic, or anaerobic adhesives now call their products ‘structural urethane,’ ‘structural acrylic,’ or ‘structural anaerobic’ adhesives. Recently, this usage has further escalated, and these products are now called ‘second generation’ or ‘third generation’ structural adhesives’’ [9, pp. 133-134].
a-Cyanoacrylates were discovered in 1949, but ‘‘the adhesive properties of a-cya — noacrylates were first recognized during the investigation of a series of 1,1-disubstituted ethylenes, in the laboratories of Eastman Kodak’’ [20, p. I]. Cyanoacrylate adhesives were first offered commercially by Eastman Kodak, their developer, in 1958 [9, p. 305].
Nylon epoxy adhesives were developed in the early 1960s. These extremely ‘‘tough’’ adhesives were used to laminate helicopter rotor blades and in honeycomb core-to-skin bonding [9, p. 157].
Urethane sealants were first used in in-plant assembly applications. ‘‘In 1960 the
two-component urethane sealants were introduced to the construction industry_____ The
properties of the urethanes, in general, are intermediate between the polysulfides and the
silicones__ The two names, ‘urethane’ and ‘polyurethane,’ are both used when referring
to this class of sealants ’’ [11, p. 93].
Thermoplastic rubber block copolymers, with completely new adhesive performance, were developed in 1965 [21]. The first commercial product was Shell Chemical’s Kraton 101, of styrene-polybutadiene-styrene composition. This development led to the carboxy — terminated nitrile (CTBN) rubber modifiers used to flexibilize epoxy and other brittle resin adhesives in the late 1960s. Today, the thermoplastic rubber block copolymer adhesives are used in hot melt-, solvent — and water-based adhesives, and as hot melt — and solvent — based sealants. Major applications are as pressure-sensitive adhesives, construction adhesives and sealants, and general assembly adhesives.
Polymercaptan sealants were commercialized in 1969. ‘‘The polymercaptans are a
new group of sealants just entering the sealant market______ The polymercaptans, with
respect to their properties, are intermediate between the polysu1fides and the urethanes’’ [11, p. 102].
Some adhesive-based resins are also used as additives, modifiers, or curing agents in other adhesive formulations. For example, starch is used in urea and phenol-formaldehyde adhesives as an extender. Poly(vinyl alcohol) is often combined with poly(vinyl acetate) to control solubility in warm or cold water in paper adhesives of the type used in schools. Polyamide higher-molecular-weight resins were first used commercially as solid hot-melt adhesives for leather shoe bonding in 1953, while the lower-molecular-weight liquid resins were first used in the mid-1950s as curing agents for epoxy resin adhesives. Today, many base resins are combined at the molecular level by the raw material suppliers. This is the case with acrylics, which have been combined with many other polymers to provide a large number of specialty resins for specific customers and applications.
In recent years a technique has been developed in which large quantities of adhesive resins called compatibilizers are used between layers of noncompatible extruded films, primarily in packaging applications. With this technology, up to seven layers of film (foil, paper, or plastic), up to three of which may be adhesive, may be combined to offer properties unlike that of any single-film material. The adhesive layer(s) may also contribute special properties to the multilayer composite film, in addition to acting as the compatibilizer. It is interesting to note that a particular plastic resin with adhesive properties may be used alone as an extruded plastic film, or in a multilayer composite film, where no compatibilizer is required, in which case it should not be counted in the adhesive statistics. This example is just one of hundreds where an intermediate or final user makes a decision to use a product sold as an adhesive, or a product sold as a nonadhesive as an adhesive, which make difficult the job of an analyst compiling industry statistics. Other common examples are the use of products labeled as coatings, encapsulants, dipping or potting compounds, modified concrete, paints, solvents, tar, thermoplastics or thermoset resins in many forms, varnishes, wheat and other flour, and so on, as adhesives. Conversely, products labeled as adhesives are often used as coatings or for other applications.
Today, even in the most developed countries, natural adhesives dominate the market because they are less expensive than synthetic-based materials, and they perform the intended function. Natural rubber is still the most widely used base material in pressure-sensitive adhesives. The first such modern uses were ‘‘flypaper’’ to trap flying insects, and medical bandages and tapes. Because of restrictions on the use of pesticides in many countries, both natural rubber and ‘‘sticky’’ synthetic materials have returned full circle to one of their original uses in trapping rodents and other small mammals. Natural rubber solvent solution adhesives are widely used throughout the world as general — purpose adhesives.
It is important to note that many adhesive technologists include brazing, soldering, and welding of metals as adhesive bonding [1, Chap. 10]. ‘‘Welding, brazing, soldering and
gluing have flow processes as a common denominator____ Soldering is a true adhesive
bonding method, as the flow process is restricted to the metallic adhesive’’ [2, p. 2]. This is not as unreasonable as might be thought, as a close study of the subjects show that there is much in common with other hot-melt joining of materials. It is just that the temperatures are often higher with the metals. However, indium and other low-temperature-melting metal alloys are often used interchangeably and at temperatures comparable to those of thermoplastic synthetic-resin hot-melt adhesives in joining metal to themselves and to other materials. FEP Teflon (copolymer of tetrafluoroethylene and hexafluoropro — pylene) and other high-temperature-melting thermoplastics are used today as hot-melt adhesives at temperatures equivalent to or exceeding those used for ordinary metal solders.
Inorganic adhesives and cements are also often classified differently by various experts. For example, sodium silicate is always classified as an adhesive when used in smaller quantities for bonding in electrical and electronic applications and for bonding paper and corrugated paperboard. But when used in larger quantities in furnace construction, they may be grouped with the portland and other hydraulic cements used in construction. Again, this makes it difficult to compile industry statistics. In the United States, the Department of Commerce has a Standard Industrial Classification (SIC) system for statistical purposes in which hydraulic cements are listed with stone, clay, and glass products as SIC 3241. Under chemicals and allied products are listed adhesives and sealants as SIC 2891 (including silicates) and dental adhesives under SIC 2844. One other class of materials, not usually considered as adhesives, that are used in large quantities worldwide for bonding thermoplastics are organic solvents. When they contain dissolved polymers, all adhesive technologists consider them as plastic cements. But when used alone, they are usually left out of the literature and the statistics. The best modern reference to their use as adhesives is Chapter 8 in the Adhesives Technology Handbook [22].
The history of the modern adhesives and sealants industry is closely tied to the development of the aircraft and aerospace industries. From the earliest flights to the most modem aerospace equipment, light weight has been one of the most vital considerations. Adhesive bonding was an ideal joining method for the early wood and textile aircraft, and today is the most important joining method for aluminium, titanium, and other metals in advanced military air — and spacecraft and some advanced commercial airplanes.
|
|
Except for a very few very high-temperature brazed panels, all honeycomb panels are adhesive bonded.
Figure 1 shows the approximate maximum service temperatures for adhesives and the approximate year of introduction [23]. The maximum service temperatures of the highest-temperature adhesives are not indicative of usefulness for prolonged exposure at these temperatures but show that systems are available for certain applications. These adhesives tend to be brittle rather than tough and are usually much more difficult to apply than are the lower-temperature systems. Other newer adhesives are usually considered experimental rather than production systems.
One of the more interesting uses of modern adhesives and sealants is by museums in the repair and restoration of antiquities. Nitrocellulose-based adhesives are widely used in such applications, as are epoxies and polyurethanes. In the United States, the Guggenheim Museum has made exhaustive, expensive, and highly scientific evaluations of the effects on durability of such repairs on irreplaceable artifacts from the past. Thus adhesives and sealants have come full circle back to some of their original uses.
1. R. Houwink and G. Salomon, eds., Adhesion and Adhesives, Vol. 1, 2nd ed., Elsevier, New York, 1965.
2. Felix Braude, Adhesives, Chemical Publishing Co., Brooklyn, N. Y., 1943.
3. Norbert M. Bikales, ed., Adhesion and Bonding, Wiley-Interscience, New York, 1971.
4. F. Smith, The Chemistry of Plant Gums and Mucilage, Reinhold, New York, 1959.
5. N. A. De Bruyne and R. Houwink, eds., Adhesion and Adhesives, Elsevier, London, 1951.
6. John Delmonte, The Technology of Adhesives, Reinhold, New York, 1947.
7. S. Buchan, Rubber to Metal Bonding, Lockwood, London 1959.
8. Adhesion and Adhesives: Fundamentals and Practice, Society of Chemical Industry, London, and Wiley, New York, 1954.
9. Gerald, L. Schneberger, ed., Adhesives in Manufacturing, Marcel Dekker, New York 1983.
10. Adolfus Damusis, ed., Sealants, Reinhold, New York, 1967.
11. John Philip Cook, Construction Sealants and Adhesives, Wiley-Interscience, New York, 1970.
12. F. A. Keimel, in Kirk-Othmer Encyclopedia of Chemical Technology, Vol. 1, 3rd ed., Wiley, New York, 1978.
13. ASTM Symposium on Plastics, Feb. 22-23, 1944.
14. Robert L. Patrick, Treatise on Adhesion and Adhesives, Vol. 2, Materials, Marcel Dekker, New York, 1969.
15. Bernard A. Dombrow, Polyurethanes, 2nd ed., Reinhold, New York, 1965.
16. Edwin P. Plueddemann, Silane Coupling Agents, Plenum Press, New York, 1982.
17. Lieng-Huang Lee, ed., Adhesion Science and Technology, Plenum Press, New York, 1975.
18. Robert S. Willard, Adhesive Patents 1955-1963, Padric Publishing, Mountainside, N. J., 1964.
19. E. Patrick McGuire, ed., Adhesives Raw Materials Handbook 1964, Padric Publishing, Mountainside, N. J., 1964.
20. Henry Lee, ed., Cyanoacrylate Resins: The Instant Adhesives, Pasadena Technology Press, Pasadena, C. A., 1981.
21. J. T. Harlan, Jr., U. S. patent 3,239,478 (1965).
22. Arthur H. Landrock, Adhesives Technology Handbook, Noyes Publications, Park Ridge, N. J., 1985.
23. H. Schwartz, Structural adhesives development, in Treatise on Adhesion and Adhesives, Vol. 4, Structural Adhesives (National Materials Advisory Board, National Research Council, ed.), Marcel Dekker, New York, 1976.
 16 июня, 2015
16 июня, 2015  Malyar
Malyar 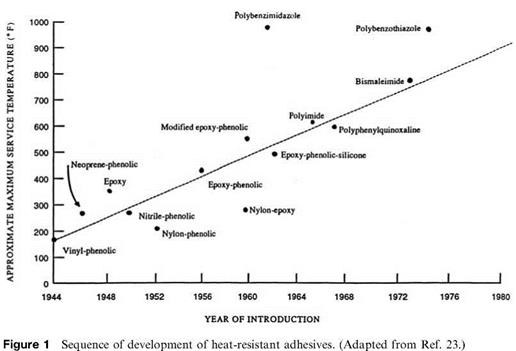
 Опубликовано в рубрике
Опубликовано в рубрике 