The adherend, adhesive, and interphase between them are major factors in determining bond durability. For example, the simple disruption of the dispersive forces already described indicates that joints made with composite adherends will be inherently more stable than those made with metallic adherends. To increase durability, most metallic and many polymeric adherends undergo surface treatments designed to alter the surface chemistry or morphology to promote primary covalent chemical bonds and/or physical bonds (mechanical keying or interlocking) to maximize, supplement, or replace secondary dispersive bonds. These treatments are discussed elsewhere [1,3,12,18-24]. An intent of each treatment is to provide interfacial bonding that is resistant to moisture intrusion.
Formation of durable chemical bonds is an obvious means to stabilize the interface and has been demonstrated for phenolic/alumina joints [25] and for silane coupling agents [26,27]. However, for most structural joints using epoxy adhesives and metallic adherends, moisture-resistant chemical bonds are not formed and mechanical interlocking on a microscopic scale is needed between the adhesive/primer and adherend for good durability. In these cases, even if moisture disrupts interfacial chemical bonds, a crack cannot follow the convoluted interface between the polymer and oxide and the joint remains intact unless this interface or the polymer itself is destroyed.
The scale of the microscopic surface roughness is important to assure good mechanical interlocking and good durability. Although roughness on any scale serves to increase the effective surface area of the adherend and, therefore, to increase the number of primary and secondary bonds with the adhesive/primer, surfaces with features on the order of a few nanometers exhibit superior performance to those with features on the order of micrometers. Several factors contribute to this difference in performance. The larger scale features are fewer in number and generally are smoother (even on a relative scale) so that interlocking is less effective. Depending on the particular treatment used, there may also be loosely bound detritus that prevents bonding to the integral adherend surface [28]. In addition, the larger scale roughness frequently allows trapped air and surface contaminants to remain at the bottoms of troughs and pores [28,29]. These unbonded regions limit joint performance by reducing both chemical and physical bonds and serving as stress concentrators. In contrast, smaller scale microroughness tends to be more convoluted in morphology and generates strong capillary forces as the primer wets the surface, drawing the polymer into all the ‘‘nooks and crannies’’ of the oxide and displacing trapped air and some contaminants to form a microcomposite interphase [29]. Indeed, cross-sectional micrographs show complete filling of the micropores [4,6,12,18,28,30].
|
Figure 1 Wedge test results for Ti adherends with several different surface treatments having differing degrees and scales of roughness. Specimens were exposed to 100% relative humidity at 60° C. (Data from Ref. 31.) |
This dependence on the degree and scale of roughness is illustrated in Fig. 1, which shows wedge test results of titanium bonds with several surface preparations [31]. Because the titanium surface is stable under these conditions (see below), differences in the joint performance can be attributed solely to differences in the polymer-to-oxide bonds and correlate very well with adherend roughness. The poorest performing group of pretreatments [Class I: phosphate fluoride (PF) and modified phosphate fluoride (MPF)] produced relatively smooth surfaces. The intermediate group [Class II: Dapcotreat (DA), dry Pasa Jell (DP), liquid Pasa Jell (LP), and Turco (TU)] exhibited macrorough surfaces with no microroughness. They had significant improvements in durability over the smooth adher — ends, but not as good as the Class III pretreatment [chromic acid anodization (CAA)] which provided a very complex microroughness. Subsequent Class III tests using sodium hydroxide anodization (SHA) and plasma spray provide further evidence of this correlation [32-34]. Both of these give very good durability performance and exhibit high levels of microroughness.
 9 июля, 2015
9 июля, 2015  Malyar
Malyar 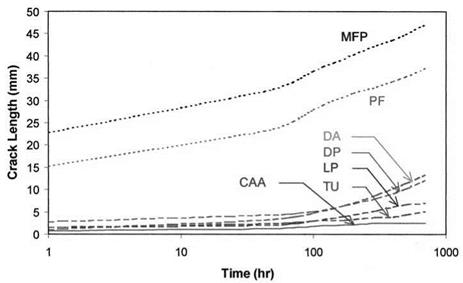
 Опубликовано в рубрике
Опубликовано в рубрике 