The strength of an adhesive joint is influenced by several factors [2-4]. Removal of contaminants and process aids provides a means for the adhesive to interlock with the substrate surface rather than with a boundary layer that is merely resting on the surface. Increasing the surface energy of the substrate above the surface tension of the adhesive makes it possible for the adhesive to wet the entire surface of the polymer substrate. The increase in the apparent surface area of contact serves to increase the strength of the adhesive bond. Figure 2 illustrates this process.
Ablation of the surface layers of the exposed polymer can result in a microroughened surface that increases the area of contact between the adhesive and the substrate. Finally, modification of the surface chemistry in a manner that facilitates covalent bonding
|
Before Plasma Treatment After Plasma Treatment Figure 2 Effect of plasma treatment on surface wetting. |
between the adhesive and the substrate surface further enhances adhesion strength. These changes are accomplished by competing molecular reactions that take place on the surface of a polymer substrate in a plasma.
1. Ablation: removal by evaporation of surface material. Ablation is the key process by which contaminants are removed from the surface of materials placed in a plasma. As the molecular weight of the contaminants is reduced due to chain scission, they become volatile enough to be removed by the vacuum system. Ablation of the surface layers of the polymer can also take place in a plasma and occur through a similar mechanism. If the substrate consists of a blend or alloy of materials that react differently in a plasma, differential ablation of these components can be used to create a microroughened surface.
2. Activation: act of substituting atoms in the polymer molecule with chemical groups from the plasma. The surface energy of the polymer placed in a plasma can be increased very rapidly by plasma-induced oxidation, nitration, hydrolization, or amination. The higher surface energy of the polymer surface increases its wettability, which describes the ability of a liquid to spread over and penetrate the surface. The increase in apparent bonded surface area that results serves to increase the strength of the bond. The process of activation can also be used to substitute surface polymer groups with those that facilitate covalent bonding between the polymer substrate and the adhesive.
 30 июня, 2015
30 июня, 2015  Malyar
Malyar 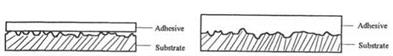
 Опубликовано в рубрике
Опубликовано в рубрике 