A. Formulation of Phenol-Resorcinol-Formaldehyde Cold-Set Adhesives
Resorcinol-based adhesives are used extensively to bond structural grade, exterior laminated beams for building construction (see Chap. 29 on Resorcinol Adhesives). The coldsetting adhesives which dominate this field are based on phenol-resorcinol-formaldehyde (PRF) resins. The adhesive itself is composed of the PRF resin and a hardener that includes formaldehyde, often in the form of paraformaldehyde mixed with inert fillers. The performance of the resin is resorcinol dependent. The cost of the resin is also resorcinol dependent as this is a very expensive chemical produced industrially in only three locations in the world. The research work on these resins from their inception has then been based on the optimization of their bonding performance coupled with the decrease in the relative percentages of resorcinol used. It has the been a long and successful work of empirical research and development which still continues.
It is, however, simpler to calculate from basic principles of polycondensation gel theory, hence using polymer chemistry, the relative amount of resorcinol which needs to be used in the preparation of a certain PRF resin to optimize its performance. As a simplified real example of the approach, let us propose the following problem.
Let us assume that we have prepared a linear phenol-formaldehyde (PF) resol resin of number average degree of polymerization DPn = 2 and average content of hydroxyben — zyl alcohol (methylol) — CH2OH groups of 2. If one wants to prepare a PRF cold-set adhesive for wood laminated beams or fingerjointing, how can one determine the minimum quantity of expensive resorcinol which is needed to make sure that the addition does not gel the resin? Such a problem in short asks what is the minimum amount of resorcinol one needs to have a good performance resin which does not gel on resorcinol addition to the reaction. A resin that is taken as hardening only on addition of further formaldehyde hardener, but only in the glue mix.
According to Eqs. (1)-(4) to assure that the resin system does not gel on addition of resorcinol one imposes the limiting condition
Pgel (experimental) > 1 (20)
As Flory’s version of the polycondensation gel theory, which we will use for this problem, underestimates by approximately 10% the degree of conversion p at the gel point this means that the limiting condition expressed by Eq. (20) can be written using Flory’s theory as
Pgel(Flory’s) > 0.9 (21)
As the functionality of resorcinol is 3 (three potential reactive sites) and of the PF resol resin is 2 (namely two reactive — CH2OH groups) this means that Flory’s branching coefficient a = 1/(f — 1) = 1(3 — 1) = 0.5, and because from Eq. (9) pj;el = a/r one can write numerically the limiting condition defined by the problem as:
0.5
(0.9)2 = — (22)
r
one obtains from this a value of r = 0.617. Consequently, because 1molPF = 2 mol — CH2OH and 1 mol resorcinol = 3 mol reactive sites:
(!)x=r (23) hence (2/3)x = 0.617 and the unknown variable x = 0.93. This means then that 0.93 moles of PF are needed for each mole of resorcinol, which is the equivalent to saying that the PF: resorcinol molar ratio > 1:1.07 is needed.
However, (3/2)x = r = 0.617 is an equally valid situation (as r is still maintained lower than 1). Solving this equation one obtains x = 0.411. This means that there will be no gelling of the resin system when adding less than 0.411 mol resorcinol to each mol PF resin, thus when the PF:resorcinol molar ratio is < 0.411.
This means then
|
|
It is then easy to determine by polymer chemistry calculations what should be the most adequate amount of resorcinol which needs to be added to a PF resin to obtain a
PRF. Three types of back-checks are, however, needed, namely:
(i) To check mathematically that the assumption that has been imposed at the beginning, pgel (Flory’s) > 0.9, is indeed correct. The 1.07 mol resorcinol is equal to 1.07 x 3 = 3.21 mol resorcinol reactive sites. According to Flory’s Theory in the most simple form pgel = (a/r)1/2, and as a = 0.5 and r = 2/3.21 = 0.623, pgel = 0.896. This is sufficiently close to the value of 0.9 which was imposed on the system at the beginning. It is then not necessary to repeat the calculation. Had this value been further from the initial 0.9 assumption a second iteration could have been done by imposing as a new starting hypothesis pgel(Flory’s) > the new value found (here 0.896), until a sufficiently stable value and condition has been found by iteration.
(ii) Visual check: what does it really mean that amounts of resorcinol lower than 0.411 mol and greater than 1.07 mol stop the system from gelling (until a hardener is added), and how can this be visualized? When the amount of resorcinol is between 0.411 mol and 1.07 mol there is sufficient resorcinol for two or more — CH2OH methylol groups from separate PF resol chains to condense on the resorcinol nuclei. This leads to the formation of a three-dimensional network sufficiently large to yield a gel: the resorcinol functions then as the ‘‘hardener’’ for the PF resol resin, as shown by the following schematic figure:
-CH2-(Phenol-CH2)„ -R-CH2 — (Phenol-CH2)n-
CH2 -(Phenol — CH2 )n-
When instead the amount of resorcinol > 1.07 mol the majority of-CH2OH methylol groups have each mainly (but not only) reacted with one resorcinol molecule. Thus, there are no more methylol groups available for reaction, if one had nearer to 2 mol resorcinol to 1 mol for the particular PF at hand. At best one methylol group reacts with one resorcinol, for example
R — CH2 -(Phenol — CH2 )n — R and R — CH2 -(Phenol — CH2)n — R
In reality the situation that presents itself is as follows
-CH2 — (Phenol — CH2)n — R — CH2 — (Phenol — CH2)n — R and
R — CH2 — (Phenol — CH2)n — R — CH2 — (Phenol — CH2)n-
This is closer to the real situation, in which PRF oligomers are formed but there is enough resorcinol present to stop the oligomers growing to too long a polymer, hence to gel. If viscosity is too high, some unreacted resorcinol is added to decrease the average molecular mass of the system. This brings the system viscosity down to a more acceptable level. No interactions are possible unless a hardener (generally additional formaldehyde) is added. This means that, in the absence of a hardener, bridges between two resorcinol nuclei grafted onto PF chains cannot form. Equally, the proportion of resorcinol is not sufficiently low for resorcinol to function as a bridge between two methylol groups of separate PF chains. In both cases formation of a very long, continuous polymer chain (the Flory equivalent of a gel) is severely inhibited. Thus, the system cannot gel unless additional formaldehyde hardener is added (which is only added in the glue mix just before use of the adhesive).
When instead the amount of resorcinol is < 0.411 mol of PF there are not sufficient molecules of resorcinol to function as a bridge between two or three methylol groups of different PF chains. The proportion of resorcinol molecules to achieve this is then insufficient for the gel portion of the system to predominate. Only short linear structures of the type
HOCH2 — (Phenol — CH2)n — R — CH2 — (Phenol — CH2)n — OH
are formed. Since the sol fraction is still in the majority, the resin system cannot gel.
(iii) Industrial formulation check: how much resorcinol is used in the best industrial PRF cold-setting adhesive formulations (most of which present a number average degree of polymerization of the original PF resol of approximately 2, and two reactive methylols)? It is interesting that the majority of the industrial PRF adhesives of this type use a PF : resorcinol : molar ratio of 1: 1.15-1: 1.17. This is an excess of just 0.08-0.1 mol resorcinol, thus just a small safety margin on what is really necessary to minimize the amount of resorcinol, maintain the adhesive’s performance, and avoid gelling. This value has also been obtained over many years by empirical research. For different PRF adhesive formulations one can redo this calculation to account for the different characteristics of the formulation and arrive at an equally correct answer without spending years of empirical research to arrive at an optimized solution.
Exactly the same type of approach can be used for the formulation of other polycondensation adhesives. For example, the formulation of melamine-urea-formaldehyde adhesive resins for wood panel products can also be successfully approached in the same way as has been shown above for the cold-setting PRF adhesives.
 29 июня, 2015
29 июня, 2015  Malyar
Malyar 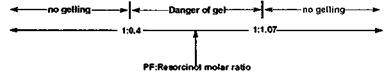
 Опубликовано в рубрике
Опубликовано в рубрике 