1. Principles
The three important techniques in this category that are discussed are XPS (also frequently called ESCA), AES, and SIMS. The basic principles of these techniques are discussed only superficially. Recent literature on these three techniques with examples of applications to materials science problems is abundant [3-14]. The surface analysis technique ion scattering spectroscopy (ISS), frequently discussed along with XPS, AES, and SIMS, is not considered in this chapter. Excellent recent reviews of this technique are available [15,16].
(a) XPS and AES. The principles of XPS are summarized in Fig. 1. Essentially a solid surface is ionized by low-energy x-rays, e. g., AlKa of 1486.6 eV. The photoelectrons emitted by the surface are collected and their energy distribution analyzed. The characteristic peaks observed in the photoelectron spectrum represent the various electron orbitals with binding energies lower than the exciting x-ray energy and are therefore specific for the elements present in the surface layers. The electrons in the energy region of interest have a mean free path in solids in the range 5-20 A. The sampling depth is roughly three times this length, i. e., 15-60 A. The technique can detect all elements with the exception of hydrogen, and an important feature of XPS is that the photoelectron peaks can shift somewhat. These shifts are dependent on the chemical state of the element. The technique, therefore, can give a quantitative analysis of all elements >H in the outermost 60 A of the sample and provides some information on the functional groups or oxidation states of these elements. Some molecular structure information can be derived from a detailed
|
|
Intensity (orb. units ) |
|
Figure 1 (Continued) |
analysis of the valence band of the material, which, however, has very low intensity. A more detailed analysis of the valence band structure is possible in UPS.
XPS has been in use since the early 1970s. Currently there are five manufacturers of commercial instruments. The strengths of XPS are its ease of operation, its quantification, and its applicability to a wide range of materials and sample forms (powders, wires, foils, chunks, etc.); in addition, both conductors and insulators can be analyzed. Sample preparation is minimal. Since the sampling depth is several dozens of monolayers, the technique is not so sensitive to surface contamination as the ion-based techniques SIMS and ISS. The major limitations of the technique are its rather poor spatial resolution (although recent improvements have been made, pushing the resolution on some commercial instruments to about 10 mm), the rather low detection limits (about 0.1 at.-% for many elements, requiring many hours of data acquisition), and the rather limited chemical information obtainable on organic materials, which is nowhere near that of, for instance, NMR and FTIR.
AES differs from XPS in that the surface is ionized by a finely focused electron beam of 5-30 kV (Fig. 1). The secondary electrons have no specific information content in AES, but the Auger electrons, which are emitted shortly after the secondary electrons, and which involve transitions between different orbitals, are recorded as a function of their kinetic energy. Such Auger electrons are also emitted after ionization by x-rays in XPS and consequently the same Auger transitions lines are also observed in XPS. In XPS, this aspect of the technique is sometimes referred to as XAES (Table 1). Because of a very high background, AES spectra are conveniently represented as differentiated spectra. The peak-to-peak heights (more accurately, peak areas in the nondifferentiated spectrum) are proportional to the number of atoms in the probed sample volume.
Since the electron energy ranges are approximately the same in XPS and AES, the surface sensitivities and sampling depths in these two techniques are very similar. In both techniques, quantification is performed, in a first approximation, by dividing the areas under the peaks (or the peak heights) by the appropriate sensitivity factor for the elements, followed by normalizing to 100%. Sensitivity factors are usually provided by the equipment manufacturer and they have been determined experimentally, although they can be calculated using ionization cross-sections (in XPS) and back scattering factors (AES). The use of these standard sets of sensitivity factors enables atomic concentrations to be determined with an accuracy of about 1-5% [14,17]. Since the electrons detected for the various elements in a sample differ in energy, the depths from which their signals originate, and hence for which their concentrations are calculated, are not the same for all elements detected. This applies to both XPS and AES.
The strength of AES lies in its small spot analysis capability. Modern instruments equipped with field emission electron guns have a spot size of about 100 A. The lateral resolution for mapping elemental distributions is therefore less than 0.1 mm, i. e., considerably better than in EDXA. The reason for this is that in AES the signal stems primarily from the surface layers; hence the broadening of the primary beam, as occurs in EDXA, does not affect the resolution in AES very much. Another capability of AES is to provide elemental concentration depth profiles using a simultaneous sputtering process by energetic ions (usually Ar+). Depth profiling can be automated under computer control. In XPS depth profiling can also be done but only intermittently, and another problem is that electrons are collected from a much wider area than in AES and, therefore, edge effects due to nonhomogeneous sputtering rates across the width of the argon beams are more likely to occur.
There are several limitations in AES. The most important one is that spectra cannot be collected (or can be collected with great difficulty) from insulating materials. Charge neutralization procedures that can be applied routinely do not exist. As opposed to surface charging in SIMS (see below), the charge in AES is of a negative sign. This limits the application of AES to metals, semiconductors, or thin films (e. g., oxides). Other limitations are the electron beam damage that easily occurs with certain materials, especially organic films, and the rather limited chemical information that can be extracted from AES spectra [18]. In principle, chemical shifts occur as in XPS, but they are more complicated because several orbitals are involved in each Auger transition and in most commercial instruments the energy resolution is not good enough to resolve such shifts. By the same token, peak overlap occurs in certain cases, especially in the energy range where many transition elements have their major AES lines, such as the range Mn-Zn (600-1000 eV). As an example, Mn in steel is very difficult to resolve and materials containing Fe, Ni, and Co would require sophisticated peak subtraction software to analyze. Other limitations are that quantification is less reliable than XPS as a result of electron back — scattering phenomena, which can be estimated but not with high accuracy. Further, the ionization probability by the primary electron beam depends on the energy of these primary electrons. In the derivative mode, errors can be introduced because peak shapes change with chemical state of the elements. This problem can be resolved, however, by determining the peak areas before spectra differentiation and by using sensitivity factors derived for the nondifferentiated peaks. Similar to XPS, the detection limits in AES are not very low, i. e., for most elements of the order of 0.1 at.-% or worse.
Current developments in AES are mainly in the areas of improved electron guns with higher brightness and smaller spot sizes, and multichannel detectors with improved sensitivities. Improvements of energy resolution will enable chemical states to be studied in more detail, leading to better analysis of complex mixtures with partly overlapping peaks.
(b) SIMS. The major ion beam technique that is currently going through a period of rapid development is SIMS [4-9]. There are several variations of the technique (Table 1), but the principle common to all is that a solid surface is bombarded by energetic ions. Ions that are commonly used are Ar+, O2+, O“, Cs+, Ga+, and others. Their energy can be in the range 5-30 keV. The impact of these ions results in the emission of secondary ions, neutral atoms, molecular fragments, and electrons. A mass spectrometer collects the ions (positive or negative) in the form of a mass spectrum. The major variations of the SIMS technique are the following:
Static SIMS. In this form of SIMS, the total primary ion dose is so low (i. e., around 1012 ions/cm2, or even less) that in the course of the experiment (1-5 min) all primary ions impinge on a fresh surface; the result is that the mass spectrum does not change with time and represents a fragmentation pattern that can be taken as a fingerprint of the material [19,20]. Both small and large ions (up to the molecular ion, or oligomers, if present) are emitted [21-23]. The sampling depth of this technique is not more than 1-2 monolayers (« 5 A). SSIMS is unique in that it detects all elements (including hydrogen) and at the same time provides molecular information on the outermost surface layers of the sample. For example, it can easily distinguish polyethylene from polypropylene (as shown in Fig. 2), detects inorganic contaminants in these polymers, and also indicates surface oxidation from the presence of O-containing ions. The latter aspect is useful for studying surface modifications of polymers by plasma or corona techniques [11,24]. For elemental detection, the technique is very sensitive, especially for alkali metals (ppm level). Although the technique is primarily used in a qualitative mode, quantitative correlations between peak ratios and elemental concentrations in XPS of the same sample have been demonstrated [25,26].
|
Figure 2 Positive quadrupole SIMS spectrum of surface of (a) polyethylene and (b) polypropylene. (From Ref. 20.) |
Dynamic SIMS. In this version of SIMS, the total ion dose is much higher than in static SIMS, up to a factor of 104. Therefore, sputtering is now the dominant process and an elemental depth profile is obtained [27]. Organic structural information is no longer contained in the spectra, because organics decompose to the elements and CHx fragments. Therefore, concentration depth profiling of organic materials, with retention of some of the molecular structure, is impossible. However, some information can be obtained if one of the constituents is specifically isotopically labeled, for instance by deuterium. As in AES, the profiling process can be automated. As a result of the lower sampling depth in SIMS, the depth resolution is better than in AES. On the other hand, the quantification of elemental concentrations is more complicated in SIMS because ion emission is strongly matrix-dependent.
Imaging SIMS. In this variation, the ion beam is rastered across a surface and a two — dimensional distribution of elements or organic materials is obtained. If this version is combined with the static SIMS mode, mapping of each peak observed in the spectrum can be performed, so even in mixtures of many organic compounds each component can be individually mapped as long as the component has at least one specific peak in the spectrum. For metals, oxides, and semiconductors, the mapping capabilities are similar to that of AES, although the sensitivity for many elements in SIMS is much higher. An example of mapping of an organic compound is shown in Fig. 3.
|
Figure 3 Time-of-Flight SIMS map of the distribution of the intensity of the ion -255 amu originating from stearic acid in lubricant residue at the surface of a cold-rolled steel sample; magnification approximately 500 x; primary ions 69Ga+ of 25 kV. |
Secondary Neutrals Mass Spectrometry. This version of SIMS detects specifically the neutral molecules or atoms that are emitted in the SIMS process [12,13]. The positionization of these neutrals is performed by low-energy electrons or by lasers used in a resonant or nonresonant mode. Since in SIMS about 99% of the emitted species are neutral particles, the positionization process increases the sensitivity of certain elements. The species that had originally been emitted as ions are, of course, detected also, but they constitute only a small fraction of the total signal. The importance of SNMS is that matrix effects are largely eliminated. In general, the emission of a species (i. e., charged and uncharged) does not depend on the chemical state or the matrix, but on the sputtering coefficient only. Peak intensities can therefore be more easily converted to concentrations using sensitivity factors only. SNMS can thus be expected to become the foremost technique for quantitative depth profiling in the near future, because it is fast, very sensitive, quantitative, and has superior depth resolution.
For static SIMS of organic materials, postionization (in this case using lasers in a nonresonant mode) can also be performed. Compared with the normal SIMS spectrum, the spectrum then contains several extra peaks if postionization is applied. These are due to the monomeric repeating unit of the polymer or to entire small molecules that are emitted. These extra ions are very useful for the identification and characterization of the materials.
Time-of-Flight SIMS. The introduction of TOF analyzers, along with postionization, is one of the major developments in the SIMS techniques of the last few years.
TOFSIMS is essentially static SIMS in which the quadrupole mass spectrometer has been replaced with a time-of-flight spectrometer, which gives the technique unique capabilities [28,29]. Ions are extracted at high voltage (e. g., 3 kV) and then enter into a field-free flight tube 1-2 meters in length where they are separated according to their flight time, which depends on their mass (Fig. 4). Advantages of this type of SIMS are that both the transmission and the mass resolution of the mass analyzer are considerably higher than
Primary Beam 5yilem primary і’ооз pulit width ipat diameter intensity mou resolution TOF-Analyter:
tl Ik live (light path acceleration voltage angular acceptance
|
Figure 5 Part of positive, high mass-resolution Time-of-Flight SIMS spectrum of a polished 304L stainless steel surface; primary ions 69Ga+ of 25 kV. |
those of the quadrupole analyzer. As a consequence, TOFSIMS has higher sensitivity (so that spectra can be recorded with lower total ion dose) and a mass resolution that enables peaks with nominally the same mass to be separated. The actual resolution obtained depends on the quality of the ion gun, which has to deliver pulses at the nanosecond level rather than being run continuously, as in quadrupole SIMS.
Because of the high mass accuracy, peak identities can now also be identified uniquely. An example is given in Fig. 5. This capability has removed most of the guesswork from SIMS analysis and has opened up many applications in materials science that are impossible in quadrupole SIMS because of peak overlap. For instance, studies of most practical metals, e. g., Al, are very difficult in quadrupole SIMS because the metal ions of interest almost always overlap with organic ions at nominally the same mass. Another important advantage of TOFSIMS is the much higher mass range than in quadrupole SIMS, which enables very large ions to be detected. An example is illustrated in Fig. 6, which shows the distribution of oligomers present in the surface of a polymer sample [23]. However, such results cannot be obtained with bulk materials. The polymer has to be present as an extremely thin film on an active metal (usually Ag). In the SIMS process the oligomers, which are emitted as neutral molecules, then become cationized by Ag+ ions.
The spectra in TOFSIMS are similar to those in quadrupole SIMS, but not identical. The time-of-flight analyzer can detect ions with a much greater kinetic energy spread than the quadrupole. Therefore, inorganic ions or low-mass organic fragments, such as C+ and CH+, which are emitted with high kinetic energies, appear in higher intensities in TOFSIMS spectra than in quadrupole spectra.
TOFSIMS is rapidly gaining popularity as a tool for studying chemistry and orientation at organic surfaces, such as polymers and polymer blends. The problem of surface
ІШЦЩцщуі
2000 3000 4000 5000 6000 7000 8000 9000
moss [omu]
Figure 6 Positive Time-of-Flight SIMS spectrum obtained from a thin film of polydimethyl — siloxane on a silver substrate showing the polymer fragmentation in the 0-500 amu range and the oligomer distribution in the higher mass ranges. (From Ref. 7.) charging (the surface charges up positively as a result of electron emission) has been satisfactorily solved by the development of pulsed electron sources, which neutralize the charge. Imaging (as shown in Fig. 3) can be performed routinely with high sensitivity and sub-micron resolution. A limitation of the technique is, however, that standard spectra of many materials are not yet available. Although much can be derived from the chemistry of the material (if known!) and the exact mass of the ions, in many cases it is not possible to identify exactly the composition or structure of the material. Much more work needs to be done in this area before the technique can be applied routinely by unskilled analysts. Fortunately, several databases (for quadrupole SIMS) have been published [19,20] and others (for TOFSIMS) are being prepared.
 17 июля, 2015
17 июля, 2015  Malyar
Malyar 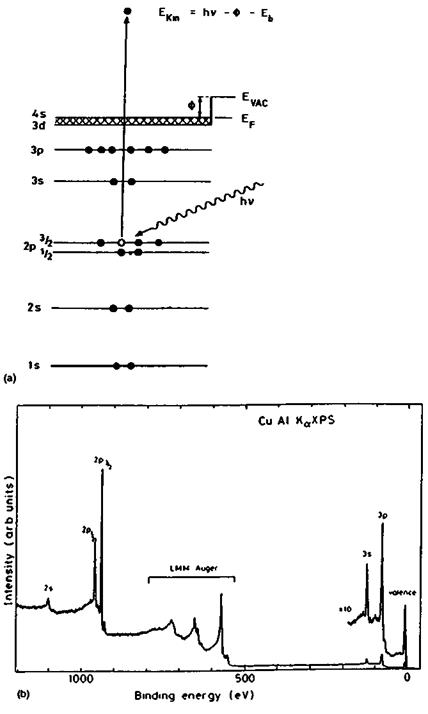
 Опубликовано в рубрике
Опубликовано в рубрике 