Quantitative results can be obtained by converting the sample compartment of a DTA apparatus into a differential calorimeter. The instrument, a differential scanning calorimeter, is built based on this principle. In this setup, the sample and reference are heated directly with separate heating coils as shown in Fig. 21. A heating coil makes the temperature of the reference material increase at a constant rate. A second heating coil is placed in the sample. The sample and the reference are kept at equal temperature. When a phase change or weight loss occurs, the sample and reference temperature become slightly different, which generates a current in the thermocouple system measuring the temperature difference between the two cells. The current activates a relay, causing extra power to be directed to the cell at the lower temperature. In this manner the temperatures of the reference and sample cells are kept virtually equal throughout. The quantity of electrical energy used in heating the sample and the reference is measured accurately and
|
|
continuously. In turn, the electrical energy is an exact measure of the number of calories used in heating the cells.
The resultant thermogram is similar to a DTA trace but more accurate and reliable. Endothermic changes are recorded as heat input into the sample, and exothermic changes as heat input into the reference. The area of the peaks is an exact measure of heat input involved. Differences in heat capacity or thermal conductivity do not affect the results. From the data, accurate quantitative analytical results can be obtained.
Much information about molecular ordering can be obtained from DSC, including the glass transition temperature (Tg), melting temperature (Tm), heat of fusion, and entropy of fusion (see Fig. 18). The melting behavior in DSC permits a determination of the extent of crystallinity. In the measurement of heats of reactions for the types of polymerization under discussion DSC is the more frequently used technique because it gives a quantitative measure of the heat and the rate of the curing reaction. All thermosetting adhesives liberate heat during cure:
Reactants ——! Products (16)
where —AH is the exothermic heat per mole of the reacting groups. Since DSC measures heat flow (dH/dt) directly it is ideally suited to measure not only the heat of reaction but also the rate of heat evolution. If the cure reaction is the only thermal event in the curing process, the reaction rate da/dt is directly proportional to dH/dt:
d a (dH /dt)
~dt = AH ( )
where at is the extent of reaction and AHt the heat evolved up to time t [57].
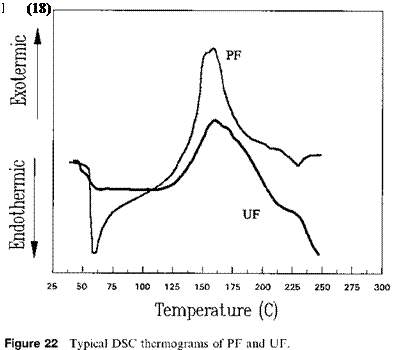
Figure 22 illustrates typical DSC thermograms for UF and PF resins. The PF resin had larger exothermic heat of curing as compared to the UF resin. For illustration purposes, the reaction between phenol and formaldehyde in alkaline conditions to form PF adhesives is briefly discussed here. The first step is an exothermic addition reaction forming methylol derivatives at the ortho or para positions:
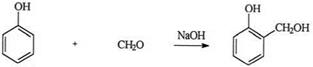
 |
(19)
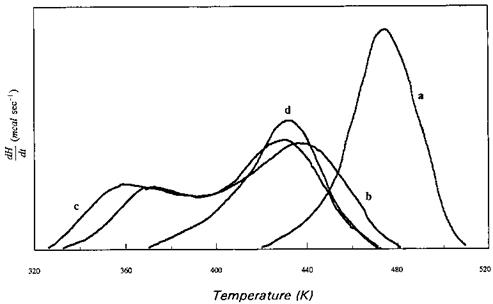
This change or transition can be monitored by DSC as shown in Fig. 23. Detailed study of curing of PF by DSC was performed by Sebenik et al. [58].
Fava [59] described kinetic measurements involving the heat of reaction for measuring the extent of cure in an epoxy resin. The three methods for obtaining isothermal cure curves using the DSC technique are isothermal operation, analysis of thermograms with different scan rates, and scans on partly cured resins. From DSC curves, the state of cure can be monitored and the kinetic parameters of cure can be determined. Using this technique, kinetic studies have been made of polymerization of vinyl acetate and PF, and curing of epoxy resins. A kinetic study of isothermal cure of epoxy resin has been carried out [60,61]. Kinetic parameters associated with the cross-linking process of PF and melamine-formaldehyde copolymers have been obtained from exotherms of a single DSC
|
Figure 23 Thermograms for the reaction between phenol and formaldehyde: (a) no catalyst added; (b) 0.25% NaOH; (c) 0.75% NaOH; (d) 5% NaOH. |
temperature scan [62-64]. The other major application of DSC is the measurement of Tg [65,66]. In the absence of endothermic or exothermic reactions, the DSC heat flow output is proportional to the sample heat capacity, and the Tg may be determined from the characteristic discontinuity in heat capacity. The Tg of a cross-linked polymer in general shows an increase with increasing degree of cross-linking, and thus provides a useful index of the degree of cure. The Tg is dependent on the chain flexibility and the free volume associated with the chemical structure as well as the overall cross-link density.
 10 июля, 2015
10 июля, 2015  Malyar
Malyar 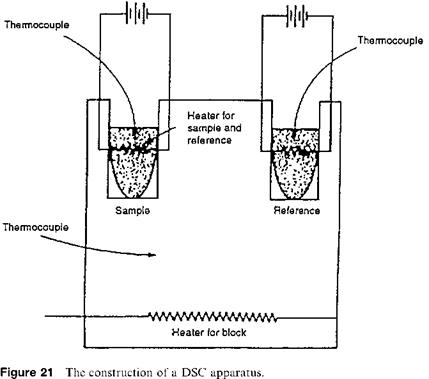
 Опубликовано в рубрике
Опубликовано в рубрике 