The IEPS (or PZC) has been defined above in the Section II. F. This acid-base property of metal oxides can be one of the key parameters controlling the adhesion properties of polymer/metal assemblies. However, because of surface rearrangement phenomena (e. g., hydration) involving the overlayers of the oxides, it is expected that siginificant differences between the surface and the bulk features of the oxides may occur. This is the reason why XPS is a very interesting technique for the assessment of the IEPS or PZC. There are three different approaches to estimate the IEPS by XPS:
(i) monitoring the uptake of ionic species by the metal oxide surface, the so-called ‘‘method of Simmons and Beard’’;
(ii) chemical shifts of the core-shell electrons from the metal oxides;
(iii) Fermi level shift monitoring.
a. The Method of Simmons and Beard. The hydroxyl groups of a hydrated oxide surface in the presence of an aqueous solution may act as an acid or a base by release or uptake of protons [153]. This can be expressed by the following equilibria and
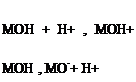 |
||
corresponding constants:
where M is the metal, K1 and K2 the acidity constants, and «MOH+, «MOH, and «MO — the surface concentrations of the different forms of the hydrated metal oxide. The PZC or the IEPS (in the absence of specific adsorption) is the pH for which «MOH+ = «MO-, that is:
PZC or IEPS = pK1 + pK2 (45)
![Assessment of the Isoelectric Point of Solids of Metal Oxides Подпись: Figure 14 Determination by XPS of (a) potassium and (b) phosphate uptake per surface hydroxyl group on the oxidized iron surface as a function of pH. (Reprinted from Ref. [154].)](/img/1207/image079_0.gif) |
Simmons and Beard [153] suggested treating solid metal oxide surfaces with solutions at a given pH containing X+ cations that can be taken up by MO — groups. Monitoring the uptake of X+ by XPS leads to the determination of K2. Similarly, one can monitor the uptake of A- anions by the surface MOH+ groups in order to determine K1. Figure 14 illustrates the determination of K1 and K2 for a hydroxylated iron surface. The pK1 and
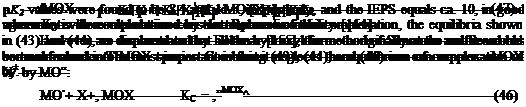
where
N = «MOX + «MOH+ + «MOH + «MO — (48)
Delamar proposed a protocol to determine IEPS values by XPS. However, his approach is yet to be checked experimentally.
b. Chemical Shifts of the Core Level Electrons from Metal Oxides. Delamar [156] used an XPS data bank and a compilation of IEPS values to establish a linear relationship between chemical shifts of the oxygen (AO) and those of the metal cations (AM) and the IEPS of the corresponding metal oxides. The chemical shifts were defined as follows:
AO = BE(O1s) — 530 eV (49)
and
AM = BE(M2p) — BE(M02p) (50)
where M is the metal in the oxidized state, M0 is the metal in the reference metallic state, and 2p is the core level shell. The value 530 eV was chosen as an arbitrary reference binding energy (BE) value for O1s. Figure 15 shows a plot of IEPS versus (AO + AM) for various metal oxides. Therefore, a simple determination of the BE shifts for a given anhydrous oxide permits estimation of its IEPS. The IEPS versus (AO + AM) correlation was confirmed by Cattania et al. [157] in their electrophoretic and XPS characterizations of a series of metal oxides having the same history.
c. Fermi Level Monitoring. An alternative approach for the assessment of the IEPS or PZC is to monitor the Fermi level (EF) shifts as proposed by Mullins and Averbach [158]. They established a correlation between EF and PZC for silica, alumina, magnesia, and phosphate powders: PZC = —2.9EF + 16.8. Clearly, the lower (higher) the Fermi level, the more basic (acidic) is the material under test. The results conform to a model of the water/oxide surface reaction that follows the generalized Lewis theory of acid-base adduct formation. They extended their approach to anodized and etched aluminum alloy surfaces [159].
Lopez et al. [160] applied the technique of Fermi level shift monitoring to characterize the acid-base properties of passive films on aluminum. The decreasing trend of relative basicity was found to be: boehmite > thermal oxide > NaOH-degreased surface > silicate containing detergent-degreased surface > phosphoric acid anodic film. The
|
Figure 15 Plot of IEPS versus (ДО+ДМ) for a series of metal oxides. ДО was defined as BE(O1s)—530 eV, where BE is the binding energy and 530 eV an arbitrary reference BE value. ДM was defined as BE(M2p)—BE(M02p) where M is the metal in the oxidized state, M0 is the metal in the reference metallic state, and 2p is the core shell. (Reprinted from Ref. [156].) |
reader is referred to [161] for further information on the background to the Fermi level monitoring.
 25 июня, 2015
25 июня, 2015  Malyar
Malyar 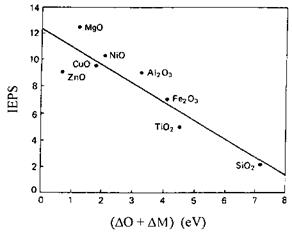
 Опубликовано в рубрике
Опубликовано в рубрике 