The effects of water and temperature on the adhesive itself are also of utmost importance to the durability of bonded structures. In the presence of moisture, the adhesive can be affected in a number of ways, depending on its chemistry and how rapidly the water permeates through and causes significant property changes [51,86-88]. The potential efficacy of moisture penetration on the locus of failure of bonded joints has been discussed in the previous section. As expected, elevated temperature conditions tend to degrade joint strength at a faster rate.
Of primary importance in moist environments is the plasticization, or softening, of the adhesive, a process that depresses Tg and lowers the modulus and strength of the elastomer [89-91]. Plasticization of the adhesive may also allow disengagement from a microrough adherend surface to reduce physical bonding and thus reduce joint strength and durability [37]. On the other hand, it may allow stress relaxation or crack blunting and improve durability [92].
Brewis et al. studied the effects of moisture and temperature on the properties of epoxy-aluminum joints by measuring changes in the mechanical strength properties of the soaked adhesive [90]. The Tg of the wet adhesive and relative strengths of wet and dry joints were evaluated for up to 2500 hours. They concluded that the joint weakening effect of water was due to plasticization of the adhesive that, in turn, was dependent on the rate of water diffusion within the adhesive.
The softening behavior has also been observed with Cytec FM 1000-Al single lap joints exposed to 100% relative humidity at 50°C for 1000 hours [91]. As shown in Fig. 8, wet and dry joints exhibited similar strength-temperature relationships, but with the former being shifted to a lower temperature by 30-50° C, a quantity close to the water — induced depression of the Tg. Hence, in this case, the Tg depression acts as a shift factor that defines the strength-temperature relationship between the dry and wet adhesive so that at a given temperature, a wet joint exhibits lower strength than a dry one.
Water entering a joint can also cause swelling, which tends to introduce stresses to weaken the bonded system. Weitsman has shown that normal stresses resulting from swelling (3%) of an epoxide adhesive are manifested at the edges of the joint; however, after an initial rise, the stress concentration decreases with time, suggesting that they do not contribute to long-term structural weakening [93].
As discussed earlier, chemical bonds between the adhesive and adherend help to stabilize the interface and increase joint durability. Aluminum joints formed with phenolic adhesives generally exhibit better durability than those with epoxy adhesives [1,92,94]. This is partly attributable to strongly interacting phenolic and aliphatic hydroxyl groups that form stable primary chemical bonds across the interface [25,95,96]. Nonetheless, epoxy adhesives are more widely used due to their greater toughness and lower temperatures and pressures required during cure.
Silanes and other coupling agents can be applied to various substrates or incorporated into an adhesive/primer to serve as hybrid chemical bridges to increase the bonding between organic adhesive and inorganic adherend surfaces [97-100]. Such bonding
|
Figure 8 The strength of wet and dry lap joints with FM-1000 adhesive as a function of temperature: (■), dry joints; (♦), joints preconditioned for 1000 hours at 50°C and 100% relative humidity. (Data from Ref. 91.) |
increases the initial bond strength and stabilizes the interface to also increase the durability of the resulting joint. Silanes and other coupling agents are discussed in Chapter 10 in this book. Silane-based primers have been shown to be effective in increasing the environmental resistance of joints prepared from aluminum [101] and titanium [102] alloys. Plueddemann has shown that the resulting interphase can be designed for maximum water resistance by employing hydrophobic resins and coupling agents and by providing a high degree of cross-linking [103].
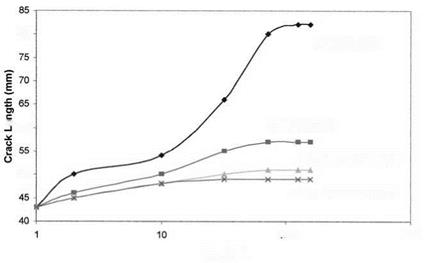
Corrosion-inhibiting adhesive primers are commonly applied onto bonding surfaces soon after the surface treatment [99]. Their primary function is to wet the adherend and penetrate the ‘‘nooks and crannies’’ to form both chemical and physical bonds. They also perform other functions essential for durable bonds: creation of a stable surface, prevention of contamination or mechanical damage of surfaces that have been chemically etched or anodized, and corrosion inhibition to the bonded and nonbonded areas of the assembly. Primer systems are normally pigmented with chromates, such as calcium, strontium, or zinc, which provide corrosion inhibition. More recent, environmentally safer primers have replaced chromates with other corrosion inhibitors. The mechanism of inhibition by chromates and some other inhibitors in the presence of moisture involves the passivation of the aluminum surface and the prevention of cathodic evolution of hydrogen by the reduction of hexavalent chromium to the trivalent state [104]. Figures 9 and 10 illustrate the improvement in joint performance using a corrosion preventative primer (Cytec BR-127) and a hydration inhibitor [12,18].
A hybrid surface treatment/primer sol-gel process has been developed that provides a graded interphase between the metal and the adhesive [78,80,81,105,106]. The sol-gel coating is typically 0.5-2 pm thick and consists of an inorganic component, an organic component, and, in some cases, a coupling agent. The inorganic component is concentrated at the metal surface (aluminum, titanium, and steel adherends have been used) while the organic component and the coupling agent are concentrated at the adhesive
|
|
|
|
|
|
|
|
|
|
|
|
|
![]()
Time (hr)
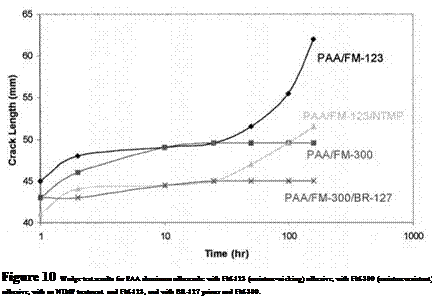
Figure 9 Wedge test results for FPL-etched aluminum adherends: with FM-123 (moisture — wicking) adhesive, with FM-300 (moisture-resistant) adhesive, with an NTMP treatment and FM-123, and with BR-127 primer and FM-300.
or conventional primer (if used). Both abraded and otherwise cleaned metal surfaces and surfaces with conventional surface treatments, such as described above, have been used. In this system, strong covalent bonds that are resistant to moisture attack are formed between the metal and the inorganic component and between the polymer and the organic component. Accordingly, physical bonding (mechanical interlocking) is less important and
these surfaces tend to be smoother than the intentionally microrough oxides normally grown on the metal surfaces. Excellent bond durability results have been reported under moisture conditions and elevated temperatures (titanium). Because the sol-gel process does not involve toxic materials, such as hexavalent chromium, or strong acids or bases, it has the potential to be an environmentally friendly surface treatment.
 9 июля, 2015
9 июля, 2015  Malyar
Malyar 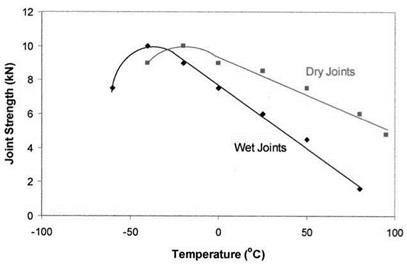
 Опубликовано в рубрике
Опубликовано в рубрике 